Minoxidil (oral)
 | |
 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration | Oral / topical |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Primarily hepatic |
| Elimination half-life | 4.2 hours |
| Excretion | renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
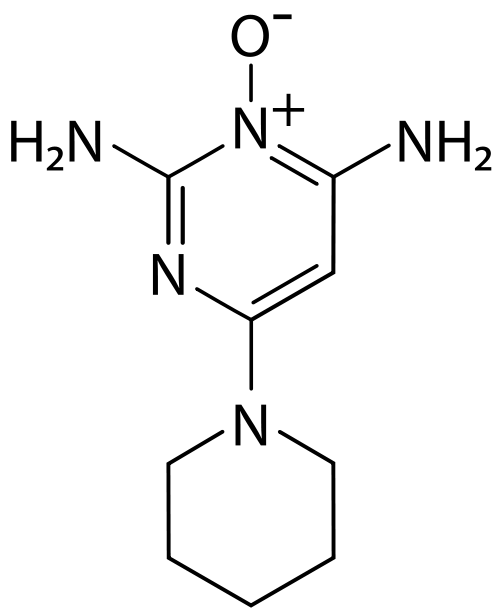
| Formula | C9H15N5O |
| Molar mass | 209.251 g/mol |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [2]
For patient information on oral Minoxidil, click here
For patient information on topical Minoxidil, click here
Minoxidil is a vasodilator and was exclusively used as an oral drug (Loniten®) to treat high blood pressure. It was, however, discovered to have the interesting side effect of hair growth and reversing baldness, and in the 1980s, Upjohn Corporation produced a topical solution that contained 2% minoxidil to be used to treat baldness and hair loss, under the brand name Rogaine in the United States, and Regaine outside the United States. Treatments usually include a 5% concentration solutions that are designed for men, while the 2% concentration solutions are designed for women. It is unknown how the drug stimulates hair growth.
In 2007 a novel, foam based formulation of 5% Minoxidil was shown to be an effective treatment of androgenetic alopecia without the usual side effects of the topical solution. [1]
Minoxidil is a "potassium channel agonist." It contains the chemical structure of nitric oxide (NO), a blood vessel dilator, and may be a nitric oxide agonist. This may explain minoxidil's ability to stimulate hair growth and treat hair loss. Since minoxidil is a nitric oxide related compound it was suspected to act via activation of guanylate cyclase, an enzyme involved in vasodilation, however there are no reports of cGMP or PKG activation to date. [2]
The patent on minoxidil expired on February 13, 1996.[3]
Side effects
As a drug to combat hair loss, the most common side effect is itchy scalp. In some cases minoxidil may initially cause an increase in hair loss.
There have been cases of allergic reactions to minoxidil or the non-active ingredient propylene glycol which is found in some forms of the topical version, such as Rogaine. Large amounts of minoxidil can cause hypotension, and it has been found that using petroleum jelly or tretinoin on the scalp with minoxidil can cause too much of the drug absorption by the scalp, as can using the drug on sunburned scalps.
If a person uses minoxidil to stop hair loss for a length of time and then stops taking the drug, hair loss will occur again.
Other side effects include:
- acne on the area where it is being used as a topical solution
- headaches and/or lightheadedness
- very low blood pressure
- irregular or fast heart beat
- blurred vision
- chest pain
All the side effects in the above list except for acne may be an indicator that too much of the drug is being used.
It has also been found that the drug can be passed from a mother to a child via breast milk.
See also
References
- ↑ Olsen EA, Whiting D, Bergfeld W, Miller J, Hordinsky M, Wanser R, Zhang P,
Kohut B (2007). "A multicenter, randomized, placebo-controlled, double-blind clinical trial of a
novel formulation of 5% minoxidil topical foam versus placebo in the treatment
of androgenetic alopecia in men". J Am Acad Dermatol. PMID 17761356. line feed character in
|author=at position 75 (help); line feed character in|title=at position 80 (help) - ↑ "Alopecia & Free Radical "Redox" Signaling--Nitric Oxide and Superoxide".
- ↑ [1]
Additional Resources
- Pages with script errors
- CS1 errors: invisible characters
- CS1 maint: Multiple names: authors list
- Drugs with non-standard legal status
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Hair loss
- Piperidines
- Pyrimidines
- Vasodilators
- Drugs