Bromocriptine: Difference between revisions
No edit summary |
m (Protected "Bromocriptine": Bot: Protecting all pages from category Drug ([Edit=Allow only administrators] (indefinite) [Move=Allow only administrators] (indefinite))) |
||
| (11 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
{{DrugProjectFormSinglePage | {{DrugProjectFormSinglePage | ||
|authorTag= | |authorTag={{VP}}<!--Overview--> | ||
|aOrAn=a | |||
{{VP}} | |drugClass=[[dopamine]] agonist | ||
|indicationType=treatment | |||
<!--Overview--> | |indication=[[acromegaly]], [[hyperprolactinemia]], and [[parkinson's disease]] | ||
|adverseReactions=[[hypotension]], [[constipation]], [[diarrhea]], [[indigestion]], [[nausea]], [[vomiting]], [[asthenia]], [[dizziness]], [[headache]], [[somnolence]], [[amblyopia]], [[rhinitis]], [[sinusitis]], and [[fatigue]] | |||
|aOrAn= | |||
a | |||
|drugClass= | |||
| | |||
| | |||
|adverseReactions= | |||
<!--Black Box Warning--> | <!--Black Box Warning--> | ||
|blackBoxWarningTitle=Title | |||
|blackBoxWarningTitle= | |blackBoxWarningBody=<i><span style="color:#FF0000;">ConditionName: </span></i> | ||
Title | |||
|blackBoxWarningBody= | |||
<i><span style="color:#FF0000;">ConditionName: </span></i> | |||
* Content | * Content | ||
| Line 41: | Line 16: | ||
<!--FDA-Labeled Indications and Dosage (Adult)--> | <!--FDA-Labeled Indications and Dosage (Adult)--> | ||
|fdaLIADAdult======Hyperprolactinemic States===== | |||
|fdaLIADAdult= | |||
=====Hyperprolactinemic States===== | |||
*The initial dosage of bromocriptine mesylate tablets in adults is ½ to one 2.5 mg scored tablet daily. An additional 2.5 mg tablet may be added to the treatment regimen as tolerated every 2-7 days until an optimal therapeutic response is achieved. The therapeutic dosage ranged from 2.5-15 mg daily in adults studied clinically. | *The initial dosage of bromocriptine mesylate tablets in adults is ½ to one 2.5 mg scored tablet daily. An additional 2.5 mg tablet may be added to the treatment regimen as tolerated every 2-7 days until an optimal therapeutic response is achieved. The therapeutic dosage ranged from 2.5-15 mg daily in adults studied clinically. | ||
*In order to reduce the likelihood of prolonged exposure to bromocriptine mesylate tablets should an unsuspected pregnancy occur, a mechanical contraceptive should be used in conjunction with bromocriptine mesylate tablets therapy until normal ovulatory menstrual cycles have been restored. Contraception may then be discontinued in patients desiring pregnancy. | *In order to reduce the likelihood of prolonged exposure to bromocriptine mesylate tablets should an unsuspected pregnancy occur, a mechanical contraceptive should be used in conjunction with bromocriptine mesylate tablets therapy until normal ovulatory menstrual cycles have been restored. Contraception may then be discontinued in patients desiring pregnancy. | ||
*Thereafter, if menstruation does not occur within 3 days of the expected date, bromocriptine mesylate therapy should be discontinued and a pregnancy test performed. | *Thereafter, if [[menstruation]] does not occur within 3 days of the expected date, bromocriptine mesylate therapy should be discontinued and a pregnancy test performed. | ||
=====Acromegaly===== | =====Acromegaly===== | ||
*Virtually all acromegalic patients receiving therapeutic benefit from bromocriptine mesylate tablets also have reductions in circulating levels of growth hormone. Therefore, periodic assessment of circulating levels of growth hormone will, in most cases, serve as a guide in determining the therapeutic potential of bromocriptine mesylate tablets. If, after a brief trial with bromocriptine mesylate therapy, no significant reduction in growth hormone levels has taken place, careful assessment of the clinical features of the disease should be made, and if no change has occurred, dosage adjustment or discontinuation of therapy should be considered. | *Virtually all [[acromegalic]] patients receiving therapeutic benefit from bromocriptine mesylate tablets also have reductions in circulating levels of growth hormone. Therefore, periodic assessment of circulating levels of [[growth hormone]] will, in most cases, serve as a guide in determining the therapeutic potential of bromocriptine mesylate tablets. If, after a brief trial with bromocriptine mesylate therapy, no significant reduction in [[growth hormone]] levels has taken place, careful assessment of the clinical features of the disease should be made, and if no change has occurred, dosage adjustment or discontinuation of therapy should be considered. | ||
*The initial recommended dosage is ½ to one 2.5 mg bromocriptine mesylate tablet on retiring (with food) for 3 days. An additional ½ to 1 tablet should be added to the treatment regimen as tolerated every 3-7 days until the patient obtains optimal therapeutic benefit. Patients should be reevaluated monthly and the dosage adjusted based on reductions of growth hormone or clinical response. The usual optimal therapeutic dosage range of bromocriptine mesylate tablets varies from 20-30 mg/day in most patients. The maximal dosage should not exceed 100 mg/day. | *The initial recommended dosage is ½ to one 2.5 mg bromocriptine mesylate tablet on retiring (with food) for 3 days. An additional ½ to 1 tablet should be added to the treatment regimen as tolerated every 3-7 days until the patient obtains optimal therapeutic benefit. Patients should be reevaluated monthly and the dosage adjusted based on reductions of [[growth hormone]] or clinical response. The usual optimal therapeutic dosage range of bromocriptine mesylate tablets varies from 20-30 mg/day in most patients. The maximal dosage should not exceed 100 mg/day. | ||
*Patients treated with pituitary irradiation should be withdrawn from bromocriptine mesylate tablets therapy on a yearly basis to assess both the clinical effects of radiation on the disease process as well as the effects of bromocriptine mesylate tablets therapy. Usually a 4-8 week withdrawal period is adequate for this purpose. Recurrence of the signs/symptoms or increases in growth hormone indicate the disease process is still active and further courses of bromocriptine mesylate tablets should be considered. | *Patients treated with pituitary irradiation should be withdrawn from bromocriptine mesylate tablets therapy on a yearly basis to assess both the clinical effects of radiation on the disease process as well as the effects of bromocriptine mesylate tablets therapy. Usually a 4-8 week withdrawal period is adequate for this purpose. Recurrence of the signs/symptoms or increases in [[growth hormone]] indicate the disease process is still active and further courses of bromocriptine mesylate tablets should be considered. | ||
=====Parkinson's Disease===== | =====Parkinson's Disease===== | ||
*The basic principle of bromocriptine mesylate tablets therapy is to initiate treatment at a low dosage and, on an individual basis, increase the daily dosage slowly until a maximum therapeutic response is achieved. The dosage of levodopa during this introductory period should be maintained, if possible. The initial dose of bromocriptine mesylate is ½ of a 2.5 mg tablet twice daily with meals. Assessments are advised at 2-week intervals during dosage titration to ensure that the lowest dosage producing an optimal therapeutic response is not exceeded. If necessary, the dosage may be increased every 14-28 days by 2½ mg/day with meals. Should it be advisable to reduce the dosage of levodopa because of adverse reactions, the daily dosage of bromocriptine mesylate, if increased, should be accomplished gradually in small (2½ mg) increments. | *The basic principle of bromocriptine mesylate tablets therapy is to initiate treatment at a low dosage and, on an individual basis, increase the daily dosage slowly until a maximum therapeutic response is achieved. The dosage of [[levodopa]] during this introductory period should be maintained, if possible. The initial dose of bromocriptine mesylate is ½ of a 2.5 mg tablet twice daily with meals. Assessments are advised at 2-week intervals during dosage titration to ensure that the lowest dosage producing an optimal therapeutic response is not exceeded. If necessary, the dosage may be increased every 14-28 days by 2½ mg/day with meals. Should it be advisable to reduce the dosage of [[levodopa]] because of adverse reactions, the daily dosage of bromocriptine mesylate, if increased, should be accomplished gradually in small (2½ mg) increments. | ||
*The safety of bromocriptine mesylate has not been demonstrated in dosages exceeding 100 mg/day. | *The safety of bromocriptine mesylate has not been demonstrated in dosages exceeding 100 mg/day. | ||
| Line 71: | Line 41: | ||
<!--Guideline-Supported Use (Adult)--> | <!--Guideline-Supported Use (Adult)--> | ||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
|offLabelAdultGuideSupport= | |||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
<!--Non–Guideline-Supported Use (Adult)--> | <!--Non–Guideline-Supported Use (Adult)--> | ||
|offLabelAdultNoGuideSupport======Female infertility - In vitro fertilization===== | |||
| | *Bromocriptine initially at 1.25 milligrams daily (mg/day) between days 4 to 6 of the follicular phase, then at 2.5 mg/day until 3 days after onset of menstruation.<ref name="pmid8690115">{{cite journal| author=Jinno M, Yoshimura Y, Ubukata Y, Nakamura Y| title=A novel method of ovarian stimulation for in vitro fertilization: bromocriptine-rebound method. | journal=Fertil Steril | year= 1996 | volume= 66 | issue= 2 | pages= 271-4 | pmid=8690115 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=8690115 }} </ref> | ||
===== | |||
: | |||
<!--Pediatric Indications and Dosage--> | <!--Pediatric Indications and Dosage--> | ||
<!--FDA-Labeled Indications and Dosage (Pediatric)--> | <!--FDA-Labeled Indications and Dosage (Pediatric)--> | ||
|fdaLIADPed======Hyperprolactinemic States===== | |||
*Based on limited data in children of age 11 to 15, the initial dose is ½ to one 2.5 mg scored tablet daily. Dosing may need to be increased as tolerated until a therapeutic response is achieved. The therapeutic dosage ranged from 2.5-10 mg daily in children with [[prolactin]]-secreting [[pituitary adenoma]]s. | |||
* Dosing | |||
<!--Off-Label Use and Dosage (Pediatric)--> | <!--Off-Label Use and Dosage (Pediatric)--> | ||
<!--Guideline-Supported Use (Pediatric)--> | <!--Guideline-Supported Use (Pediatric)--> | ||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
|offLabelPedGuideSupport= | |||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Non–Guideline-Supported Use (Pediatric)--> | <!--Non–Guideline-Supported Use (Pediatric)--> | ||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
|offLabelPedNoGuideSupport= | |||
There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Contraindications--> | <!--Contraindications--> | ||
|contraindications=*[[Hypersensitivity]] to bromocriptine or to any of the excipients of bromocriptine mesylate tablets, uncontrolled [[hypertension]] and sensitivity to any ergot alkaloids. In patients being treated for [[hyperprolactinemia]], bromocriptine mesylate should be withdrawn when pregnancy is diagnosed. In the event that bromocriptine mesylate is reinstituted to control a rapidly expanding macroadenoma and a patient experiences a hypertensive disorder of pregnancy, the benefit of continuing bromocriptine mesylate must be weighed against the possible risk of its use during a [[hypertensive]] disorder of pregnancy. | |||
*When bromocriptine mesylate is being used to treat [[acromegaly]], [[prolactinoma]], or [[Parkinson’s disease]] in patients who subsequently become pregnant, a decision should be made as to whether the therapy continues to be medically necessary or can be withdrawn. If it is continued, the drug should be withdrawn in those who may experience hypertensive disorders of pregnancy (including [[eclampsia]], [[preeclampsia]], or [[pregnancy-induced hypertension]]) unless withdrawal of bromocriptine mesylate is considered to be medically contraindicated. | |||
*The drug should not be used during the [[postpartum]] period in women with a history of [[coronary artery disease]] and other severe cardiovascular conditions unless withdrawal is considered medically contraindicated. If the drug is used in the postpartum period, the patient should be observed with caution. | |||
*The drug should not be used during the postpartum period in women with a history of coronary artery disease and other severe cardiovascular conditions unless withdrawal is considered medically contraindicated. If the drug is used in the postpartum period, the patient should be observed with caution. | |||
<!--Warnings--> | <!--Warnings--> | ||
|warnings=*Since [[hyperprolactinemia]] with [[amenorrhea]]/[[galactorrhea]] and [[infertility]] has been found in patients with [[pituitary tumors]], a complete evaluation of the pituitary is indicated before treatment with bromocriptine mesylate. | |||
|warnings= | |||
*Since hyperprolactinemia with amenorrhea/galactorrhea and infertility has been found in patients with pituitary tumors, a complete evaluation of the pituitary is indicated before treatment with bromocriptine mesylate. | |||
*If pregnancy occurs during bromocriptine mesylate administration, careful observation of these patients is mandatory. Prolactin-secreting adenomas may expand and compression of the optic or other cranial nerves may occur, emergency pituitary surgery becoming necessary. In most cases, the compression resolves following delivery. Reinitiation of bromocriptine mesylate treatment has been reported to produce improvement in the visual fields of patients in whom nerve compression has occurred during pregnancy. The safety of bromocriptine mesylate treatment during pregnancy to the mother and fetus has not been established. | *If pregnancy occurs during bromocriptine mesylate administration, careful observation of these patients is mandatory. Prolactin-secreting adenomas may expand and compression of the optic or other cranial nerves may occur, emergency pituitary surgery becoming necessary. In most cases, the compression resolves following delivery. Reinitiation of bromocriptine mesylate treatment has been reported to produce improvement in the visual fields of patients in whom nerve compression has occurred during pregnancy. The safety of bromocriptine mesylate treatment during pregnancy to the mother and fetus has not been established. | ||
*Bromocriptine mesylate has been associated with somnolence, and episodes of sudden sleep onset, particularly in patients with Parkinson’s disease. Sudden onset of sleep during daily activities, in some cases without awareness or warning signs, has been reported. Patients must be informed of this and advised not to drive or operate machines during treatment with bromocriptine. Patients who have experienced somnolence and/or an episode of sudden sleep onset must not drive or operate machines. Furthermore, a reduction of dosage or termination of therapy may be considered. | *Bromocriptine mesylate has been associated with [[somnolence]], and episodes of sudden sleep onset, particularly in patients with [[Parkinson’s disease]]. Sudden onset of sleep during daily activities, in some cases without awareness or warning signs, has been reported. Patients must be informed of this and advised not to drive or operate machines during treatment with bromocriptine. Patients who have experienced somnolence and/or an episode of sudden sleep onset must not drive or operate machines. Furthermore, a reduction of dosage or termination of therapy may be considered. | ||
*Symptomatic hypotension can occur in patients treated with bromocriptine mesylate for any indication. In postpartum studies with bromocriptine mesylate, decreases in supine systolic and diastolic pressures of greater than 20 mm and 10 mm Hg, respectively, have been observed in almost 30% of patients receiving bromocriptine mesylate. On occasion, the drop in supine systolic pressure was as much as 50-59 mm of Hg. | *Symptomatic [[hypotension]] can occur in patients treated with bromocriptine mesylate for any indication. In postpartum studies with bromocriptine mesylate, decreases in supine systolic and diastolic pressures of greater than 20 mm and 10 mm Hg, respectively, have been observed in almost 30% of patients receiving bromocriptine mesylate. On occasion, the drop in supine systolic pressure was as much as 50-59 mm of Hg. | ||
*Since, especially during the first days of treatment, hypotensive reactions may occasionally occur and result in reduced alertness, particular care should be exercised when driving a vehicle or operating machinery. | *Since, especially during the first days of treatment, hypotensive reactions may occasionally occur and result in reduced alertness, particular care should be exercised when driving a vehicle or operating machinery. | ||
*While hypotension during the start of therapy with bromocriptine mesylate occurs in some patients, in rare cases serious adverse events, including hypertension, myocardial infarction, seizures, stroke, have been reported in postpartum women treated with bromocriptine mesylate. Hypertension has been reported, sometimes at the initiation of therapy, but often developing in the second week of therapy; seizures have also been reported both with and without the prior development of hypertension; stroke has been reported mostly in postpartum patients whose prenatal and obstetric courses had been uncomplicated. Many of these patients experiencing seizures (including cases of status epilepticus) and/or strokes reported developing a constant and often progressively severe headache hours to days prior to the acute event. Some cases of strokes and seizures were also preceded by visual disturbances (blurred vision, and transient cortical blindness). Cases of acute myocardial infarction have also been reported. | *While [[hypotension]] during the start of therapy with bromocriptine mesylate occurs in some patients, in rare cases serious adverse events, including [[hypertension]], [[myocardial infarction]], [[seizures]], [[stroke]], have been reported in postpartum women treated with bromocriptine mesylate. [[Hypertension]] has been reported, sometimes at the initiation of therapy, but often developing in the second week of therapy; seizures have also been reported both with and without the prior development of [[hypertension]]; stroke has been reported mostly in postpartum patients whose prenatal and obstetric courses had been uncomplicated. Many of these patients experiencing [[seizures]] (including cases of [[status epilepticus]]) and/or strokes reported developing a constant and often progressively severe headache hours to days prior to the acute event. Some cases of strokes and [[seizures]] were also preceded by visual disturbances ([[blurred vision]], and transient [[cortical blindness]]). Cases of acute [[myocardial infarction]] have also been reported. | ||
*Although a causal relationship between bromocriptine mesylate administration and hypertension, seizures, strokes, and myocardial infarction in postpartum women has not been established, use of the drug in patients with uncontrolled hypertension is not recommended. In patients being treated for hyperprolactinemia, bromocriptine mesylate should be withdrawn when pregnancy is diagnosed | *Although a causal relationship between bromocriptine mesylate administration and [[hypertension]], [[seizures]], [[strokes]], and [[myocardial infarction]] in postpartum women has not been established, use of the drug in patients with uncontrolled hypertension is not recommended. In patients being treated for [[hyperprolactinemia]], bromocriptine mesylate should be withdrawn when pregnancy is diagnosed. In the event that bromocriptine mesylate is reinstituted to control a rapidly expanding macroadenoma and a patient experiences a hypertensive disorder of pregnancy, the benefit of continuing bromocriptine mesylate must be weighed against the possible risk of its use during a hypertensive disorder of pregnancy. When bromocriptine mesylate is being used to treat [[acromegaly]] or [[Parkinson’s disease]] in patients who subsequently become pregnant, a decision should be made as to whether the therapy continues to be medically necessary or can be withdrawn. If it is continued, the drug should be withdrawn in those who may experience hypertensive disorders of pregnancy (including [[eclampsia]], [[preeclampsia]], or pregnancy-induced hypertension) unless withdrawal of bromocriptine mesylate is considered to be medically contraindicated. Because of the possibility of an interaction between bromocriptine mesylate and other ergot alkaloids, the concomitant use of these medications is not recommended. Periodic monitoring of the blood pressure, particularly during the first weeks of therapy is prudent. If [[hypertension]], severe, progressive, or unremitting [[headache]] (with or without visual disturbance), or evidence of CNS toxicity develops, drug therapy should be discontinued and the patient should be evaluated promptly. Particular attention should be paid to patients who have recently been treated or are on concomitant therapy with drugs that can alter blood pressure. Their concomitant use in the [[puerperium]] is not recommended. | ||
*Among patients on bromocriptine mesylate, particularly on long-term and high-dose treatment, pleural and pericardial | *Among patients on bromocriptine mesylate, particularly on long-term and high-dose treatment, pleural and [[pericardial effusion]]s, as well as pleural and pulmonary fibrosis and [[constrictive pericarditis]], have been reported. Patients with unexplained pleuropulmonary disorders should be examined thoroughly and discontinuation of bromocriptine mesylate therapy should be considered. In those instances in which bromocriptine mesylate treatment was terminated, the changes slowly reverted towards normal. | ||
*In a few patients on bromocriptine mesylate, particularly on long-term and high-dose treatment, retroperitoneal fibrosis has been reported. To ensure recognition of retroperitoneal fibrosis at an early reversible stage it is recommended that its manifestations (e.g., back pain, edema of the lower limbs, impaired kidney function) should be watched in this category of patients. Bromocriptine mesylate medication should be withdrawn if fibrotic changes in the retroperitoneum are diagnosed or suspected. | *In a few patients on bromocriptine mesylate, particularly on long-term and high-dose treatment, [[retroperitoneal fibrosis]] has been reported. To ensure recognition of [[retroperitoneal fibrosis]] at an early reversible stage it is recommended that its manifestations (e.g., [[back pain]], edema of the lower limbs, impaired kidney function) should be watched in this category of patients. Bromocriptine mesylate medication should be withdrawn if fibrotic changes in the [[retroperitoneum]] are diagnosed or suspected. | ||
====Precautions==== | ====Precautions==== | ||
| Line 192: | Line 94: | ||
*General | *General | ||
:*Safety and efficacy of bromocriptine mesylate have not been established in patients with renal or hepatic disease. Care should be exercised when administering bromocriptine mesylate therapy concomitantly with other medications known to lower blood pressure. | :*Safety and efficacy of bromocriptine mesylate have not been established in patients with renal or hepatic disease. Care should be exercised when administering bromocriptine mesylate therapy concomitantly with other medications known to lower blood pressure. | ||
:*The drug should be used with caution in patients with a history of psychosis or cardiovascular disease. If acromegalic patients or patients with prolactinoma or Parkinson's disease are being treated with bromocriptine mesylate during pregnancy, they should be cautiously observed, particularly during the postpartum period if they have a history of cardiovascular disease. | :*The drug should be used with caution in patients with a history of psychosis or cardiovascular disease. If acromegalic patients or patients with [[prolactinoma]] or [[Parkinson's disease]] are being treated with bromocriptine mesylate during pregnancy, they should be cautiously observed, particularly during the postpartum period if they have a history of cardiovascular disease. | ||
:*Patients with rare hereditary problems of galactose intolerance, severe lactase deficiency or glucose-galactose malabsorption should not take this medicine. | :*Patients with rare hereditary problems of [[galactose intolerance]], severe [[lactase deficiency]] or glucose-galactose malabsorption should not take this medicine. | ||
*Hyperprolactinemic States | *Hyperprolactinemic States | ||
:*Visual field impairment is a known complication of macroprolactinoma. Effective treatment with bromocriptine mesylate leads to a reduction in hyperprolactinemia and often to a resolution of the visual impairment. In some patients, however, a secondary deterioration of visual fields may subsequently develop despite normalized prolactin levels and tumor shrinkage, which may result from traction on the optic chiasm which is pulled down into the now partially empty sella. In these cases, the visual field defect may improve on reduction of bromocriptine dosage while there is some elevation of prolactin and some tumor re-expansion. Monitoring of visual fields in patients with macroprolactinoma is therefore recommended for an early recognition of secondary field loss due to chiasmal herniation and adaptation of drug dosage. | :*Visual field impairment is a known complication of macroprolactinoma. Effective treatment with bromocriptine mesylate leads to a reduction in hyperprolactinemia and often to a resolution of the visual impairment. In some patients, however, a secondary deterioration of visual fields may subsequently develop despite normalized [[prolactin]] levels and tumor shrinkage, which may result from traction on the optic chiasm which is pulled down into the now partially empty sella. In these cases, the visual field defect may improve on reduction of bromocriptine dosage while there is some elevation of prolactin and some tumor re-expansion. Monitoring of visual fields in patients with [[macroprolactinoma]] is therefore recommended for an early recognition of secondary field loss due to chiasmal herniation and adaptation of drug dosage. | ||
:*The relative efficacy of bromocriptine mesylate versus surgery in preserving visual fields is not known. Patients with rapidly progressive visual field loss should be evaluated by a neurosurgeon to help decide on the most appropriate therapy. | :*The relative efficacy of bromocriptine mesylate versus surgery in preserving visual fields is not known. Patients with rapidly progressive visual field loss should be evaluated by a neurosurgeon to help decide on the most appropriate therapy. | ||
:*Since pregnancy is often the therapeutic objective in many hyperprolactinemic patients presenting with amenorrhea/galactorrhea and hypogonadism (infertility), a careful assessment of the pituitary is essential to detect the presence of a prolactin-secreting adenoma. Patients not seeking pregnancy, or those harboring large adenomas, should be advised to use contraceptive measures, other than oral contraceptives, during treatment with bromocriptine mesylate. Since pregnancy may occur prior to reinitiation of menses, a pregnancy test is recommended at least every 4 weeks during the amenorrheic period, and, once menses are reinitiated, every time a patient misses a menstrual period. Treatment with bromocriptine mesylate should be discontinued as soon as pregnancy has been established. Patients must be monitored closely throughout pregnancy for signs and symptoms that may signal the enlargement of a previously undetected or existing prolactin-secreting tumor. Discontinuation of bromocriptine mesylate treatment in patients with known macroadenomas has been associated with rapid regrowth of tumor and increase in serum prolactin in most cases. | :*Since pregnancy is often the therapeutic objective in many hyperprolactinemic patients presenting with [[amenorrhea]]/[[galactorrhea]] and [[hypogonadism]] ([[infertility]]), a careful assessment of the pituitary is essential to detect the presence of a prolactin-secreting adenoma. Patients not seeking pregnancy, or those harboring large adenomas, should be advised to use contraceptive measures, other than oral contraceptives, during treatment with bromocriptine mesylate. Since pregnancy may occur prior to reinitiation of menses, a pregnancy test is recommended at least every 4 weeks during the amenorrheic period, and, once menses are reinitiated, every time a patient misses a menstrual period. Treatment with bromocriptine mesylate should be discontinued as soon as pregnancy has been established. Patients must be monitored closely throughout pregnancy for signs and symptoms that may signal the enlargement of a previously undetected or existing prolactin-secreting tumor. Discontinuation of bromocriptine mesylate treatment in patients with known macroadenomas has been associated with rapid regrowth of tumor and increase in serum [[prolactin]] in most cases. | ||
:*Cerebrospinal fluid rhinorrhea has been observed in some patients with prolactin-secreting adenomas treated with bromocriptine mesylate. | :*[[Cerebrospinal fluid]] [[rhinorrhea]] has been observed in some patients with prolactin-secreting adenomas treated with bromocriptine mesylate. | ||
*Acromegaly | *Acromegaly | ||
:*Cld-sensitive digital vasospasm has been observed in some acromegalic patients treated with bromocriptine mesylate. The response, should it occur, can be reversed by reducing the dose of bromocriptine mesylate and may be prevented by keeping the fingers warm. Cases of severe gastrointestinal bleeding from peptic ulcers have been reported, some fatal. Although there is no evidence that bromocriptine mesylate increases the incidence of peptic ulcers in acromegalic patients, symptoms suggestive of peptic ulcer should be investigated thoroughly and treated appropriately. Patients with a history of peptic ulcer or gastrointestinal bleeding should be observed carefully during treatment with bromocriptine mesylate. | :*Cld-sensitive digital [[vasospasm]] has been observed in some acromegalic patients treated with bromocriptine mesylate. The response, should it occur, can be reversed by reducing the dose of bromocriptine mesylate and may be prevented by keeping the fingers warm. Cases of severe gastrointestinal bleeding from peptic ulcers have been reported, some fatal. Although there is no evidence that bromocriptine mesylate increases the incidence of peptic ulcers in acromegalic patients, symptoms suggestive of peptic ulcer should be investigated thoroughly and treated appropriately. Patients with a history of [[peptic ulcer]] or [[gastrointestinal bleeding]] should be observed carefully during treatment with bromocriptine mesylate. | ||
:*Possible tumor expansion while receiving bromocriptine mesylate therapy has been reported in a few patients. Since the natural history of growth hormone-secreting tumors is unknown, all patients should be carefully monitored and, if evidence of tumor expansion develops, discontinuation of treatment and alternative procedures considered. | :*Possible tumor expansion while receiving bromocriptine mesylate therapy has been reported in a few patients. Since the natural history of growth hormone-secreting tumors is unknown, all patients should be carefully monitored and, if evidence of tumor expansion develops, discontinuation of treatment and alternative procedures considered. | ||
*Parkinson's Disease | *Parkinson's Disease | ||
:*Safety during long-term use for more than 2 years at the doses required for parkinsonism has not been established. | :*Safety during long-term use for more than 2 years at the doses required for [[parkinsonism]] has not been established. | ||
:*As with any chronic therapy, periodic evaluation of hepatic, hematopoietic, cardiovascular, and renal function is recommended. Symptomatic hypotension can occur and, therefore, caution should be exercised when treating patients receiving antihypertensive drugs. | :*As with any chronic therapy, periodic evaluation of hepatic, hematopoietic, cardiovascular, and renal function is recommended. Symptomatic hypotension can occur and, therefore, caution should be exercised when treating patients receiving antihypertensive drugs. | ||
:*High doses of bromocriptine mesylate may be associated with confusion and mental disturbances. Since parkinsonian patients may manifest mild degrees of dementia, caution should be used when treating such patients. | :*High doses of bromocriptine mesylate may be associated with confusion and mental disturbances. Since parkinsonian patients may manifest mild degrees of dementia, caution should be used when treating such patients. | ||
:*Bromocriptine mesylate administered alone or concomitantly with levodopa may cause hallucinations (visual or auditory). Hallucinations usually resolve with dosage reduction; occasionally, discontinuation of bromocriptine mesylate is required. Rarely, after high doses, hallucinations have persisted for several weeks following discontinuation of bromocriptine mesylate. | :*Bromocriptine mesylate administered alone or concomitantly with levodopa may cause [[hallucinations]] (visual or auditory). [[Hallucinations]] usually resolve with dosage reduction; occasionally, discontinuation of bromocriptine mesylate is required. Rarely, after high doses, hallucinations have persisted for several weeks following discontinuation of bromocriptine mesylate. | ||
:*Postmarketing reports suggest that patients treated with anti-Parkinson medications can experience intense urges to gamble, increased sexual urges, intense urges to spend money uncontrollably, and other intense urges. Patients may be unable to control these urges while taking one or more of the medications that are generally used for the treatment of Parkinson’s disease and that increase central dopaminergic tone, including bromocriptine mesylate. In some cases, although not all, these urges were reported to have stopped when the dose was reduced or the medication was discontinued. Because patients may not recognize these behaviors as abnormal it is important for prescribers to specifically ask patients or their caregivers about the development of new or increased gambling urges, sexual urges, uncontrolled spending or other urges while being treated with bromocriptine mesylate. Physicians should consider dose reduction or stopping the medication if a patient develops such urges while taking bromocriptine mesylate. | :*Postmarketing reports suggest that patients treated with anti-Parkinson medications can experience intense urges to gamble, increased sexual urges, intense urges to spend money uncontrollably, and other intense urges. Patients may be unable to control these urges while taking one or more of the medications that are generally used for the treatment of [[Parkinson’s disease]] and that increase central [[dopaminergic]] tone, including bromocriptine mesylate. In some cases, although not all, these urges were reported to have stopped when the dose was reduced or the medication was discontinued. Because patients may not recognize these behaviors as abnormal it is important for prescribers to specifically ask patients or their caregivers about the development of new or increased gambling urges, sexual urges, uncontrolled spending or other urges while being treated with bromocriptine mesylate. Physicians should consider dose reduction or stopping the medication if a patient develops such urges while taking bromocriptine mesylate. | ||
:*As with levodopa, caution should be exercised when administering bromocriptine mesylate to patients with a history of myocardial infarction who have a residual atrial, nodal, or ventricular arrhythmia. | :*As with [[levodopa]], caution should be exercised when administering bromocriptine mesylate to patients with a history of [[myocardial infarction]] who have a residual atrial, nodal, or [[ventricular arrhythmia]]. | ||
:*Retroperitoneal fibrosis has been reported in a few patients receiving long-term therapy (2-10 years) with bromocriptine mesylate in doses ranging from 30-140 mg daily. | :*[[Retroperitoneal fibrosis]] has been reported in a few patients receiving long-term therapy (2-10 years) with bromocriptine mesylate in doses ranging from 30-140 mg daily. | ||
:*Epidemiological studies have shown that patients with Parkinson’s disease have a higher risk (2-approximately 6-fold higher) of developing melanoma than the general population. Whether the increased risk observed was due to Parkinson’s disease or other factors, such as drugs used to treat Parkinson’s disease, is unclear. For the reasons stated above, patients and providers are advised to monitor for melanomas frequently and on a regular basis when using bromocriptine mesylate for any indication. Ideally, periodic skin examinations should be performed by appropriately qualified individuals (e.g. dermatologists). | :*Epidemiological studies have shown that patients with [[Parkinson’s disease]] have a higher risk (2-approximately 6-fold higher) of developing [[melanoma]] than the general population. Whether the increased risk observed was due to Parkinson’s disease or other factors, such as drugs used to treat [[Parkinson’s disease]], is unclear. For the reasons stated above, patients and providers are advised to monitor for melanomas frequently and on a regular basis when using bromocriptine mesylate for any indication. Ideally, periodic skin examinations should be performed by appropriately qualified individuals (e.g. dermatologists). | ||
:*Discontinuation of bromocriptine mesylate should be undertaken gradually whenever possible, even if the patient is to remain on L-dopa. A symptom complex resembling the neuroleptic malignant syndrome (characterized by elevated temperature, muscular rigidity, altered consciousness, and autonomic instability), with no other obvious etiology, has been reported in association with rapid dose reduction, withdrawal of, or changes in antiparkinsonian therapy. | :*Discontinuation of bromocriptine mesylate should be undertaken gradually whenever possible, even if the patient is to remain on L-dopa. A symptom complex resembling the [[neuroleptic malignant syndrome]] (characterized by elevated temperature, muscular rigidity, altered consciousness, and [[autonomic instability]]), with no other obvious etiology, has been reported in association with rapid dose reduction, withdrawal of, or changes in antiparkinsonian therapy. | ||
<!--Adverse Reactions--> | <!--Adverse Reactions--> | ||
<!--Clinical Trials Experience--> | <!--Clinical Trials Experience--> | ||
|clinicalTrials======Hyperprolactinemic Indications===== | |||
*The incidence of adverse effects is quite high (69%) but these are generally mild to moderate in degree. Therapy was discontinued in approximately 5% of patients because of adverse effects. These in decreasing order of frequency are: [[nausea]] (49%), [[headache]] (19%), [[dizziness]] (17%), [[fatigue]] (7%), [[lightheadedness]] (5%), [[vomiting]] (5%), [[abdominal cramps]] (4%), nasal congestion (3%), [[constipation]] (3%), [[diarrhea]] (3%) and [[drowsiness]] (3%). | |||
*A slight hypotensive effect may accompany bromocriptine mesylate treatment. The occurrence of adverse reactions may be lessened by temporarily reducing dosage to ½ tablet 2 or 3 times daily. A few cases of [[cerebrospinal fluid]] [[rhinorrhea]] have been reported in patients receiving bromocriptine mesylate for treatment of large [[prolactinomas]]. This has occurred rarely, usually only in patients who have received previous transsphenoidal surgery, pituitary radiation, or both, and who were receiving bromocriptine mesylate for tumor recurrence. It may also occur in previously untreated patients whose tumor extends into the [[sphenoid sinus]]. | |||
*A slight hypotensive effect may accompany bromocriptine mesylate treatment. The occurrence of adverse reactions may be lessened by temporarily reducing dosage to ½ tablet 2 or 3 times daily. A few cases of cerebrospinal fluid rhinorrhea have been reported in patients receiving bromocriptine mesylate for treatment of large prolactinomas. This has occurred rarely, usually only in patients who have received previous transsphenoidal surgery, pituitary radiation, or both, and who were receiving bromocriptine mesylate for tumor recurrence. It may also occur in previously untreated patients whose tumor extends into the sphenoid sinus. | |||
=====Acromegaly===== | =====Acromegaly===== | ||
*The most frequent adverse reactions encountered in acromegalic patients treated with bromocriptine mesylate were: nausea (18%), constipation (14%), postural/orthostatic hypotension (6%), anorexia (4%), dry mouth/nasal stuffiness (4%), indigestion/dyspepsia (4%), digital vasospasm (3%), drowsiness/tiredness (3%) and vomiting (2%). | *The most frequent adverse reactions encountered in acromegalic patients treated with bromocriptine mesylate were: [[nausea]] (18%), [[constipation]] (14%), postural/[[orthostatic hypotension]] (6%), [[anorexia]] (4%), [[dry mouth]]/nasal stuffiness (4%), indigestion/[[dyspepsia]] (4%), digital [[vasospasm]] (3%), [[drowsiness]]/tiredness (3%) and [[vomiting]] (2%). | ||
*Less frequent adverse reactions (less than 2%) were: gastrointestinal bleeding, dizziness, exacerbation of Raynaud’s syndrome, headache and syncope. Rarely (less than 1%) hair loss, alcohol potentiation, faintness, lightheadedness, arrhythmia, ventricular tachycardia, decreased sleep requirement, visual hallucinations, lassitude, shortness of breath, bradycardia, vertigo, paresthesia, sluggishness, vasovagal attack, delusional psychosis, paranoia, insomnia, heavy headedness, reduced tolerance to cold, tingling of ears, facial pallor and muscle cramps have been reported. | *Less frequent adverse reactions (less than 2%) were: gastrointestinal bleeding, dizziness, exacerbation of [[Raynaud’s syndrome]], [[headache]] and [[syncope]]. Rarely (less than 1%) hair loss, alcohol potentiation, faintness, [[lightheadedness]], [[arrhythmia]], [[ventricular tachycardia]], decreased sleep requirement, visual [[hallucinations]], lassitude, [[shortness of breath]], [[bradycardia]], [[vertigo]], [[paresthesia]], sluggishness, [[vasovagal attack]], delusional [[psychosis]], [[paranoia]], [[insomnia]], heavy headedness, reduced tolerance to cold, tingling of ears, facial pallor and muscle cramps have been reported. | ||
=====Parkinson's Disease===== | =====Parkinson's Disease===== | ||
*In clinical trials in which bromocriptine mesylate was administered with concomitant reduction in the dose of levodopa/carbidopa, the most common newly appearing adverse reactions were: nausea, abnormal involuntary movements, hallucinations, confusion, “on-off’’ phenomenon, dizziness, drowsiness, faintness/fainting, vomiting, asthenia, abdominal discomfort, visual disturbance, ataxia, insomnia, depression, hypotension, shortness of breath, constipation, and vertigo. | *In clinical trials in which bromocriptine mesylate was administered with concomitant reduction in the dose of [[levodopa]]/[[carbidopa]], the most common newly appearing adverse reactions were: [[nausea]], abnormal involuntary movements, [[hallucinations]], confusion, “on-off’’ phenomenon, [[dizziness]], drowsiness, faintness/fainting, [[vomiting]], [[asthenia]], [[abdominal discomfort]], visual disturbance, [[ataxia]], [[insomnia]], depression, [[hypotension]], [[shortness of breath]], [[constipation]], and [[vertigo]]. | ||
*Less common adverse reactions which may be encountered include: anorexia, anxiety, blepharospasm, dry mouth, dysphagia, edema of the feet and ankles, erythromelalgia, epileptiform seizure, fatigue, headache, lethargy, mottling of skin, nasal stuffiness, nervousness, nightmares, paresthesia, skin rash, urinary frequency, urinary incontinence, urinary retention, and rarely, signs and symptoms of ergotism such as tingling of fingers, cold feet, numbness, muscle cramps of feet and legs or exacerbation of Raynaud’s Syndrome. | *Less common adverse reactions which may be encountered include: [[anorexia]], anxiety, [[blepharospasm]], [[dry mouth]], [[dysphagia]], edema of the feet and ankles, [[erythromelalgia]], epileptiform seizure, fatigue, [[headache]], lethargy, mottling of skin, nasal stuffiness, nervousness, nightmares, [[paresthesia]], skin [[rash]], urinary frequency, [[urinary incontinence]], urinary retention, and rarely, signs and symptoms of ergotism such as tingling of fingers, cold feet, numbness, muscle cramps of feet and legs or exacerbation of [[Raynaud’s Syndrome]]. | ||
*Abnormalities in laboratory tests may include elevations in blood urea nitrogen, SGOT, SGPT, GGPT, CPK, alkaline phosphatase and uric acid, which are usually transient and not of clinical significance. | *Abnormalities in laboratory tests may include elevations in blood urea nitrogen, SGOT, SGPT, GGPT, CPK, alkaline phosphatase and uric acid, which are usually transient and not of clinical significance. | ||
| Line 244: | Line 143: | ||
=====Postpartum Patients===== | =====Postpartum Patients===== | ||
*In postpartum studies with bromocriptine mesylate, 23 percent of postpartum patients treated had at least 1 side effect, but they were generally mild to moderate in degree. Therapy was discontinued in approximately 3% of patients. The most frequently occurring adverse reactions were: headache (10%), dizziness (8%), nausea (7%), vomiting (3%), fatigue (1.0%), syncope (0.7%), diarrhea (0.4%) and cramps (0.4%). Decreases in blood pressure (≥ 20 mm Hg systolic and ≥ 10 mm Hg diastolic) occurred in 28% of patients at least once during the first 3 postpartum days; these were usually of a transient nature. Reports of fainting in the puerperium may possibly be related to this effect. In postmarketing experience in the U.S., serious adverse reactions reported include 72 cases of seizures (including 4 cases of status epilepticus), 30 cases of stroke, and 9 cases of myocardial infarction among postpartum patients. Seizure cases were not necessarily accompanied by the development of hypertension. An unremitting and often progressively severe headache, sometimes accompanied by visual disturbance, often preceded by hours to days many cases of seizure and/or stroke. Most patients had shown no evidence of any of the hypertensive disorders of pregnancy including eclampsia, preeclampsia or pregnancy-induced hypertension. One stroke case was associated with sagittal sinus thrombosis, and another was associated with cerebral and cerebellar vasculitis. One case of myocardial infarction was associated with unexplained disseminated intravascular coagulation and a second occurred in conjunction with use of another ergot alkaloid. The relationship of these adverse reactions to bromocriptine mesylate administration has not been established. | *In postpartum studies with bromocriptine mesylate, 23 percent of postpartum patients treated had at least 1 side effect, but they were generally mild to moderate in degree. Therapy was discontinued in approximately 3% of patients. The most frequently occurring adverse reactions were: [[headache]] (10%), [[dizziness]] (8%), [[nausea]] (7%), [[vomiting]] (3%), [[fatigue]] (1.0%), [[syncope]] (0.7%), [[diarrhea]] (0.4%) and cramps (0.4%). Decreases in blood pressure (≥ 20 mm Hg systolic and ≥ 10 mm Hg diastolic) occurred in 28% of patients at least once during the first 3 postpartum days; these were usually of a transient nature. Reports of fainting in the puerperium may possibly be related to this effect. In postmarketing experience in the U.S., serious adverse reactions reported include 72 cases of [[seizures]] (including 4 cases of [[status epilepticus]]), 30 cases of [[stroke]], and 9 cases of [[myocardial infarction]] among postpartum patients. Seizure cases were not necessarily accompanied by the development of [[hypertension]]. An unremitting and often progressively severe [[headache]], sometimes accompanied by visual disturbance, often preceded by hours to days many cases of [[seizure]] and/or [[stroke]]. Most patients had shown no evidence of any of the [[hypertensive]] disorders of pregnancy including [[eclampsia]], [[preeclampsia]] or [[pregnancy-induced hypertension]]. One stroke case was associated with [[sagittal sinus thrombosis]], and another was associated with cerebral and cerebellar [[vasculitis]]. One case of [[myocardial infarction]] was associated with unexplained [[disseminated intravascular coagulation]] and a second occurred in conjunction with use of another ergot alkaloid. The relationship of these adverse reactions to bromocriptine mesylate administration has not been established. | ||
*In rare cases serious adverse events, including hypertension, myocardial infarction, seizures, stroke, or psychic disorders have been reported in postpartum women treated with bromocriptine mesylate. In some patients the development of seizures or stroke was preceded by severe headache and/or transient visual disturbances. Although the causal relationship of these events to the drug is uncertain, periodic monitoring of blood pressure is advisable in postpartum women receiving bromocriptine mesylate. If hypertension, severe, progressive, or unremitting headache (with or without visual disturbances), or evidence of CNS toxicity develop, the administration of bromocriptine mesylate should be discontinued and the patient should be evaluated promptly. | *In rare cases serious adverse events, including [[hypertension]], [[myocardial infarction]], [[seizures]], [[stroke]], or psychic disorders have been reported in postpartum women treated with bromocriptine mesylate. In some patients the development of [[seizures]] or [[stroke]] was preceded by severe [[headache]] and/or transient visual disturbances. Although the causal relationship of these events to the drug is uncertain, periodic monitoring of blood pressure is advisable in postpartum women receiving bromocriptine mesylate. If [[hypertension]], severe, progressive, or unremitting [[headache]] (with or without visual disturbances), or evidence of CNS toxicity develop, the administration of bromocriptine mesylate should be discontinued and the patient should be evaluated promptly. | ||
*Particular caution is required in patients who have recently been treated or are on concomitant therapy with drugs that can alter blood pressure, e.g. vasoconstrictors such as sympathomimetics or ergot alkaloids including ergometrine or methylergometrine and their concomitant use in the puerperium is not recommended. | *Particular caution is required in patients who have recently been treated or are on concomitant therapy with drugs that can alter blood pressure, e.g. [[vasoconstrictors]] such as [[sympathomimetics]] or ergot alkaloids including [[ergometrine]] or methylergometrine and their concomitant use in the [[puerperium]] is not recommended. | ||
<!--Postmarketing Experience--> | <!--Postmarketing Experience--> | ||
|postmarketing=*The following adverse reactions have been reported during postapproval use of bromocriptine mesylate (All Indications Combined). Because adverse reactions from spontaneous reports are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or establish a causal relationship to drug exposure. | |||
|postmarketing= | |||
*The following adverse reactions have been reported during postapproval use of bromocriptine mesylate (All Indications Combined). Because adverse reactions from spontaneous reports are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or establish a causal relationship to drug exposure. | |||
=====Psychiatric disorders===== | =====Psychiatric disorders===== | ||
Confusion, psychomotor agitation/excitation, hallucinations, psychotic disorders, insomnia, libido increase, hypersexuality. | [[Confusion]], psychomotor agitation/excitation, [[hallucinations]], psychotic disorders, [[insomnia]], [[libido]] increase, hypersexuality. | ||
=====Nervous system disorders===== | =====Nervous system disorders===== | ||
Headache, drowsiness, dizziness, dyskinaesia, somnolence, paraesthesia, excess daytime somnolence, sudden onset of sleep. | [[Headache]], [[drowsiness]], [[dizziness]], dyskinaesia, somnolence, [[paraesthesia]], excess daytime [[somnolence]], sudden onset of sleep. | ||
=====Eye disorders===== | =====Eye disorders===== | ||
Visual disturbance, vision | Visual disturbance, [[blurred vision]]. | ||
=====Ear and labyrinth disorders===== | =====Ear and labyrinth disorders===== | ||
Tinnitus. | [[Tinnitus]]. | ||
=====Cardiac disorders===== | =====Cardiac disorders===== | ||
Pericardial effusion, constrictive pericarditis, tachycardia, bradycardia, arrhythmia, cardiac valve fibrosis. | [[Pericardial effusion]], [[constrictive pericarditis]], [[tachycardia]], [[bradycardia]], [[arrhythmia]], cardiac valve fibrosis. | ||
=====Vascular disorders===== | =====Vascular disorders===== | ||
Hypotension, orthostatic hypotension (very rarely leading to syncope), reversible pallor of fingers and toes induced by cold (especially in patients with history of Raynaud's phenomenon) | [[Hypotension]], [[orthostatic hypotension]] (very rarely leading to [[syncope]]), reversible pallor of fingers and toes induced by cold (especially in patients with history of [[Raynaud's phenomenon]]) | ||
=====Respiratory, thoracic and mediastinal disorders===== | =====Respiratory, thoracic and mediastinal disorders===== | ||
Nasal congestion, pleural effusion, pleural fibrosis, pleurisy, pulmonary fibrosis, | Nasal congestion, [[pleural effusion]], pleural fibrosis, [[pleurisy]], [[pulmonary fibrosis]], [[dyspnea]]. | ||
=====Gastrointestinal disorders===== | =====Gastrointestinal disorders===== | ||
Nausea, constipation, vomiting, dry mouth, | [[Nausea]], [[constipation]], [[vomiting]], [[dry mouth]], [[diarrhea]], [[abdominal pain]], [[retroperitoneal fibrosis]], [[gastrointestinal ulcer]], [[gastrointestinal haemorrhage]]. | ||
=====Skin and subcutaneous tissue disorders===== | =====Skin and subcutaneous tissue disorders===== | ||
Allergic skin reactions, hair loss. | Allergic skin reactions, [[hair loss]]. | ||
=====Musculoskeletal and connective tissue disorders===== | =====Musculoskeletal and connective tissue disorders===== | ||
| Line 298: | Line 194: | ||
=====General disorders and administration site conditions===== | =====General disorders and administration site conditions===== | ||
Fatigue, peripheral edema, a syndrome resembling Neuroleptic Malignant Syndrome on abrupt withdrawal of bromocriptine mesylate. | [[Fatigue]], [[peripheral edema]], a syndrome resembling [[Neuroleptic Malignant Syndrome]] on abrupt withdrawal of bromocriptine mesylate. | ||
<!--Drug Interactions--> | <!--Drug Interactions--> | ||
|drugInteractions=*The risk of using bromocriptine mesylate in combination with other drugs has not been systematically evaluated, but alcohol may potentiate the side effects of bromocriptine mesylate. Bromocriptine mesylate may interact with [[dopamine]] antagonists, butyrophenones, and certain other agents. Compounds in these categories result in a decreased efficacy of bromocriptine mesylate: [[phenothiazines]], [[haloperidol]], [[metoclopramide]], [[pimozide]]. Bromocriptine is a substrate of CYP3A4. Caution should therefore be used when co-administering drugs which are strong inhibitors of this enzyme (such as azole antimycotics, HIV protease inhibitors). The concomitant use of [[macrolide]] antibiotics such as [[erythromycin]] was shown to increase the plasma levels of bromocriptine (mean AUC and Cmax values increased 3.7-fold and 4.6-fold, respectively). 1The concomitant treatment of acromegalic patients with bromocriptine and [[octreotide]] led to increased plasma levels of bromocriptine (bromocriptine AUC increased about 38%). 4Concomitant use of bromocriptine mesylate with other ergot alkaloids is not recommended. Dose adjustment may be necessary in those cases where high doses of bromocriptine are being used (such as [[Parkinson’s disease]] indication). | |||
|drugInteractions= | |||
*The risk of using bromocriptine mesylate in combination with other drugs has not been systematically evaluated, but alcohol may potentiate the side effects of bromocriptine mesylate. Bromocriptine mesylate may interact with dopamine antagonists, butyrophenones, and certain other agents. Compounds in these categories result in a decreased efficacy of bromocriptine mesylate: phenothiazines, haloperidol, metoclopramide, pimozide. Bromocriptine is a substrate of CYP3A4. Caution should therefore be used when co-administering drugs which are strong inhibitors of this enzyme (such as azole antimycotics, HIV protease inhibitors). The concomitant use of macrolide antibiotics such as erythromycin was shown to increase the plasma levels of bromocriptine (mean AUC and Cmax values increased 3.7-fold and 4.6-fold, respectively). 1The concomitant treatment of acromegalic patients with bromocriptine and octreotide led to increased plasma levels of bromocriptine (bromocriptine AUC increased about 38%). 4Concomitant use of bromocriptine mesylate with other ergot alkaloids is not recommended. Dose adjustment may be necessary in those cases where high doses of bromocriptine are being used (such as Parkinson’s disease indication). | |||
<!--Use in Specific Populations--> | <!--Use in Specific Populations--> | ||
|useInPregnancyFDA=* '''Pregnancy Category B''' | |||
|useInPregnancyFDA= | |||
* '''Pregnancy Category B''' | |||
*Administration of 10-30 mg/kg of bromocriptine to 2 strains of rats on days 6-15 postcoitum (p.c.) as well as a single dose of 10 mg/kg on day 5 p.c. interfered with nidation. | *Administration of 10-30 mg/kg of bromocriptine to 2 strains of rats on days 6-15 postcoitum (p.c.) as well as a single dose of 10 mg/kg on day 5 p.c. interfered with nidation. | ||
| Line 321: | Line 212: | ||
*Information concerning 1276 pregnancies in women taking bromocriptine mesylate has been collected. In the majority of cases, bromocriptine mesylate was discontinued within 8 weeks into pregnancy (mean 28.7 days), however, 8 patients received the drug continuously throughout pregnancy. The mean daily dose for all patients was 5.8 mg (range 1-40 mg). | *Information concerning 1276 pregnancies in women taking bromocriptine mesylate has been collected. In the majority of cases, bromocriptine mesylate was discontinued within 8 weeks into pregnancy (mean 28.7 days), however, 8 patients received the drug continuously throughout pregnancy. The mean daily dose for all patients was 5.8 mg (range 1-40 mg). | ||
*Of these 1276 pregnancies, there were 1088 full-term deliveries (4 stillborn), 145 spontaneous abortions (11.4%), and 28 induced abortions (2.2%). Moreover, 12 extrauterine gravidities and 3 hydatidiform | *Of these 1276 pregnancies, there were 1088 full-term deliveries (4 stillborn), 145 spontaneous abortions (11.4%), and 28 induced abortions (2.2%). Moreover, 12 extrauterine gravidities and 3 [[hydatidiform mole]]s (twice in the same patient) caused early termination of pregnancy. These data compare favorably with the abortion rate (11%-25%) cited for pregnancies induced by [[clomiphene citrate]], menopausal gonadotropin, and chorionic [[gonadotropin]]. | ||
*Although spontaneous abortions often go unreported, especially prior to 20 weeks of gestation, their frequency has been estimated to be 15%. | *Although spontaneous [[abortions]] often go unreported, especially prior to 20 weeks of gestation, their frequency has been estimated to be 15%. | ||
*The incidence of birth defects in the population at large ranges from 2%-4.5%. The incidence in 1109 live births from patients receiving bromocriptine is 3.3%. | *The incidence of birth defects in the population at large ranges from 2%-4.5%. The incidence in 1109 live births from patients receiving bromocriptine is 3.3%. | ||
*There is no suggestion that bromocriptine mesylate contributed to the type or incidence of birth defects in this group of infants. | *There is no suggestion that bromocriptine mesylate contributed to the type or incidence of birth defects in this group of infants. | ||
|useInPregnancyAUS=* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | |||
|useInPregnancyAUS= | |||
* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | |||
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | ||
|useInLaborDelivery=There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | |||
|useInNursing=*Bromocriptine mesylate should not be used during lactation in [[postpartum]] women. | |||
|useInPed=*The safety and effectiveness of bromocriptine for the treatment of prolactin-secreting pituitary adenomas have been established in patients age 16 to adult. No data are available for bromocriptine use in pediatric patients under the age of 8 years. A single 8-year-old patient treated with bromocriptine for a prolactin-secreting pituitary macroadenoma has been reported without therapeutic response. | |||
*The use of bromocriptine for the treatment of prolactin-secreting adenomas in pediatric patients in the age group 11 to under 16 years is supported by evidence from well-controlled trials in adults, with additional data in a limited number (n=14) of children and adolescents 11 to 15 years of age with [[prolactin]]-secreting pituitary macro- and microadenomas who have been treated with bromocriptine. Of the 14 reported patients, 9 had successful outcomes, 3 partial responses, and 2 failed to respond to bromocriptine treatment. Chronic [[hypopituitarism]] complicated [[macroadenoma]] treatment in 5 of the responders, both in patients receiving bromocriptine alone and in those who received bromocriptine in combination with surgical treatment and/or pituitary irradiation. | |||
*The use of bromocriptine for the treatment of prolactin-secreting adenomas in pediatric patients in the age group 11 to under 16 years is supported by evidence from well-controlled trials in adults, with additional data in a limited number (n=14) of children and adolescents 11 to 15 years of age with prolactin-secreting pituitary macro- and microadenomas who have been treated with bromocriptine. Of the 14 reported patients, 9 had successful outcomes, 3 partial responses, and 2 failed to respond to bromocriptine treatment. Chronic hypopituitarism complicated macroadenoma treatment in 5 of the responders, both in patients receiving bromocriptine alone and in those who received bromocriptine in combination with surgical treatment and/or pituitary irradiation. | |||
*Safety and effectiveness of bromocriptine in pediatric patients have not been established for any other indication listed in the INDICATIONS AND USAGE section. | *Safety and effectiveness of bromocriptine in pediatric patients have not been established for any other indication listed in the INDICATIONS AND USAGE section. | ||
|useInGeri=*Clinical studies for bromocriptine mesylate did not include sufficient numbers of subjects aged 65 and over to determine whether the elderly respond differently from younger subjects. However, other reported clinical experiences, including postmarketing reporting of adverse events, have not identified differences in response or tolerability between elderly and younger patients. Even though no variation in efficacy or adverse reaction profile in geriatric patients taking bromocriptine mesylate has been observed, greater sensitivity of some elderly individuals cannot be categorically ruled out. In general, dose selection for an elderly patient should be cautious, starting at the lower end of the dose range, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy in this population. | |||
|useInGeri= | |useInGender=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | ||
|useInRace=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | |||
*Clinical studies for bromocriptine mesylate did not include sufficient numbers of subjects aged 65 and over to determine whether the elderly respond differently from younger subjects. However, other reported clinical experiences, including postmarketing reporting of adverse events, have not identified differences in response or tolerability between elderly and younger patients. Even though no variation in efficacy or adverse reaction profile in geriatric patients taking bromocriptine mesylate has been observed, greater sensitivity of some elderly individuals cannot be categorically ruled out. In general, dose selection for an elderly patient should be cautious, starting at the lower end of the dose range, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy in this population. | |useInRenalImpair=There is no FDA guidance on the use of {{PAGENAME}} in patients with renal impairment. | ||
|useInHepaticImpair=There is no FDA guidance on the use of {{PAGENAME}} in patients with hepatic impairment. | |||
|useInGender= | |useInReproPotential=There is no FDA guidance on the use of {{PAGENAME}} in women of reproductive potentials and males. | ||
There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | |useInImmunocomp=There is no FDA guidance one the use of {{PAGENAME}} in patients who are immunocompromised. | ||
|useInRace= | |||
There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | |||
|useInRenalImpair= | |||
There is no FDA guidance on the use of {{PAGENAME}} in patients with renal impairment. | |||
|useInHepaticImpair= | |||
There is no FDA guidance on the use of {{PAGENAME}} in patients with hepatic impairment. | |||
|useInReproPotential= | |||
There is no FDA guidance on the use of {{PAGENAME}} in women of reproductive potentials and males. | |||
|useInImmunocomp= | |||
There is no FDA guidance one the use of {{PAGENAME}} in patients who are immunocompromised. | |||
<!--Administration and Monitoring--> | <!--Administration and Monitoring--> | ||
|administration=* Oral | |||
|administration= | |monitoring=There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | ||
* Oral | |||
|monitoring= | |||
There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | |||
<!--IV Compatibility--> | <!--IV Compatibility--> | ||
|IVCompat=There is limited information regarding <i>IV Compatibility</i> of {{PAGENAME}} in the drug label. | |||
|IVCompat= | |||
There is limited information regarding <i>IV Compatibility</i> of {{PAGENAME}} in the drug label. | |||
<!--Overdosage--> | <!--Overdosage--> | ||
|overdose====Acute Overdose=== | |||
|overdose= | |||
===Acute Overdose=== | |||
====Signs and Symptoms==== | ====Signs and Symptoms==== | ||
*The most commonly reported signs and symptoms associated with acute bromocriptine mesylate overdose are: nausea, vomiting, constipation, diaphoresis, dizziness, pallor, severe hypotension, malaise, confusion, lethargy, drowsiness, delusions, hallucinations, and repetitive yawning. The lethal dose has not been established and the drug has a very wide margin of safety. However, one death occurred in a patient who committed suicide with an unknown quantity of bromocriptine mesylate and chloroquine. | *The most commonly reported signs and symptoms associated with acute bromocriptine mesylate overdose are: [[nausea]], [[vomiting]], [[constipation]], [[diaphoresis]], [[dizziness]], [[pallor]], severe [[hypotension]], [[malaise]], confusion, lethargy, [[drowsiness]], [[delusions]], [[hallucinations]], and repetitive yawning. The lethal dose has not been established and the drug has a very wide margin of safety. However, one death occurred in a patient who committed suicide with an unknown quantity of bromocriptine mesylate and chloroquine. | ||
====Management==== | ====Management==== | ||
*Treatment of overdose consists of removal of the drug by emesis (if conscious), gastric lavage, activated charcoal, or saline catharsis. Careful supervision and recording of fluid intake and output is essential. Hypotension should be treated by placing the patient in the Trendelenburg position and administering I.V. fluids. If satisfactory relief of hypotension cannot be achieved by using the above measures to their fullest extent, vasopressors should be considered. | *Treatment of overdose consists of removal of the drug by [[emesis]] (if conscious), [[gastric lavage]], activated charcoal, or saline catharsis. Careful supervision and recording of fluid intake and output is essential. [[Hypotension]] should be treated by placing the patient in the [[Trendelenburg position]] and administering I.V. fluids. If satisfactory relief of [[hypotension]] cannot be achieved by using the above measures to their fullest extent, vasopressors should be considered. | ||
===Chronic Overdose=== | ===Chronic Overdose=== | ||
| Line 408: | Line 262: | ||
<!--Drug box 2--> | <!--Drug box 2--> | ||
|drugBox={{Drugbox2 | |||
|drugBox= | |||
{{Drugbox2 | |||
| Watchedfields = changed | | Watchedfields = changed | ||
| verifiedrevid = 459983915 | | verifiedrevid = 459983915 | ||
| Line 474: | Line 325: | ||
<!--Mechanism of Action--> | <!--Mechanism of Action--> | ||
|mechAction=* Bromocriptine mesylate is a dopamine receptor agonist, which activates post-synaptic dopamine receptors. The dopaminergic neurons in the tuberoinfundibular process modulate the secretion of prolactin from the anterior pituitary by secreting a prolactin inhibitory factor (thought to be dopamine); in the corpus striatum the dopaminergic neurons are involved in the control of motor function. Clinically, bromocriptine mesylate significantly reduces plasma levels of [[prolactin]] in patients with physiologically elevated [[prolactin]] as well as in patients with [[hyperprolactinemia]]. The inhibition of physiological lactation as well as [[galactorrhea]] in pathological hyperprolactinemic states is obtained at dose levels that do not affect secretion of other tropic hormones from the anterior pituitary. Experiments have demonstrated that bromocriptine induces long-lasting stereotyped behavior in rodents and turning behavior in rats having unilateral lesions in the [[substantia nigra]]. These actions, characteristic of those produced by [[dopamine]], are inhibited by dopamine antagonists and suggest a direct action of bromocriptine on striatal dopamine receptors. | |||
*Bromocriptine mesylate is a nonhormonal, nonestrogenic agent that inhibits the secretion of [[prolactin]] in humans, with little or no effect on other pituitary hormones, except in patients with [[acromegaly]], where it lowers elevated blood levels of growth hormone in the majority of patients. | |||
*Bromocriptine mesylate is a nonhormonal, nonestrogenic agent that inhibits the secretion of prolactin in humans, with little or no effect on other pituitary hormones, except in patients with acromegaly, where it lowers elevated blood levels of growth hormone in the majority of patients. | |||
<!--Structure--> | <!--Structure--> | ||
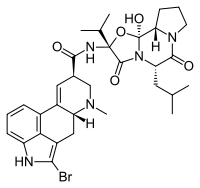
|structure=* Bromocriptine mesylate is an ergot derivative with potent dopamine receptor agonist activity. Each bromocriptine mesylate tablet, USP for oral administration contains 2.5 mg bromocriptine (as the mesylate). Bromocriptine mesylate is chemically designated as Ergotaman-3',6',18-trione, 2-bromo-12'-hydroxy-2'-(1-methylethyl)-5'-(2-methylpropyl)-,(5'α)-monomethanesulfonate (salt). | |||
|structure= | |||
* Bromocriptine mesylate is an ergot derivative with potent dopamine receptor agonist activity. Each bromocriptine mesylate tablet, USP for oral administration contains 2.5 mg bromocriptine (as the mesylate). Bromocriptine mesylate is chemically designated as Ergotaman-3',6',18-trione, 2-bromo-12'-hydroxy-2'-(1-methylethyl)-5'-(2-methylpropyl)-,(5'α)-monomethanesulfonate (salt). | |||
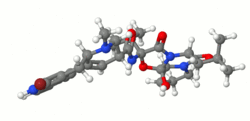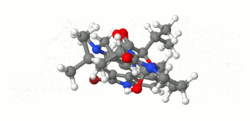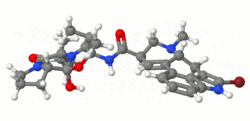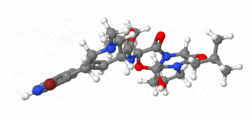
*The structural formula is: | *The structural formula is: | ||
| Line 496: | Line 341: | ||
<!--Pharmacodynamics--> | <!--Pharmacodynamics--> | ||
|PD=*Bromocriptine mesylate produces its therapeutic effect in the treatment of [[Parkinson’s disease]], a clinical condition characterized by a progressive deficiency in [[dopamine]] synthesis in the [[substantia nigra]], by directly stimulating the [[dopamine]] receptors in the corpus striatum. In contrast, [[levodopa]] exerts its therapeutic effect only after conversion to [[dopamine]] by the neurons of the substantia nigra, which are known to be numerically diminished in this patient population. | |||
|PD= | |||
*Bromocriptine mesylate produces its therapeutic effect in the treatment of Parkinson’s disease, a clinical condition characterized by a progressive deficiency in dopamine synthesis in the substantia nigra, by directly stimulating the dopamine receptors in the corpus striatum. In contrast, levodopa exerts its therapeutic effect only after conversion to dopamine by the neurons of the substantia nigra, which are known to be numerically diminished in this patient population. | |||
<!--Pharmacokinetics--> | <!--Pharmacokinetics--> | ||
|PK=*Absorption | |||
|PK= | |||
*Absorption | |||
:*Following single dose administration of bromocriptine mesylate tablets, 2 x 2.5 mg to 5 healthy volunteers under fasted conditions, the mean peak plasma levels of bromocriptine, time to reach peak plasma concentrations and elimination half-life were 465 pg/mL ± 226, 2.5 hrs ± 2 and 4.85 hr, respectively. Linear relationship was found between single doses of bromocriptine mesylate and Cmax and AUC in the dose range of 1 to 7.5 mg. The pharmacokinetics of bromocriptine metabolites have not been reported. | :*Following single dose administration of bromocriptine mesylate tablets, 2 x 2.5 mg to 5 healthy volunteers under fasted conditions, the mean peak plasma levels of bromocriptine, time to reach peak plasma concentrations and elimination half-life were 465 pg/mL ± 226, 2.5 hrs ± 2 and 4.85 hr, respectively. Linear relationship was found between single doses of bromocriptine mesylate and Cmax and AUC in the dose range of 1 to 7.5 mg. The pharmacokinetics of bromocriptine metabolites have not been reported. | ||
:*Food did not significantly affect the systemic exposure of bromocriptine following administration of bromocriptine mesylate tablets, 2.5 mg. It is recommended that bromocriptine mesylate be taken with food because of the high percentage of subjects who vomit upon receiving bromocriptine under fasting conditions. | :*Food did not significantly affect the systemic exposure of bromocriptine following administration of bromocriptine mesylate tablets, 2.5 mg. It is recommended that bromocriptine mesylate be taken with food because of the high percentage of subjects who vomit upon receiving bromocriptine under fasting conditions. | ||
| Line 511: | Line 350: | ||
*Distribution | *Distribution | ||
:*In vitro experiments showed that bromocriptine was 90%-96% bound to serum albumin. | :*In vitro experiments showed that bromocriptine was 90%-96% bound to serum [[albumin]]. | ||
*Metabolism | *Metabolism | ||
:*Bromocriptine undergoes extensive first-pass biotransformation, reflected by complex metabolite profiles and by almost complete absence of parent drug in urine and feces. | :*Bromocriptine undergoes extensive first-pass biotransformation, reflected by complex metabolite profiles and by almost complete absence of parent drug in urine and feces. | ||
:*In vitro studies using human liver microsomes showed that bromocriptine has a high affinity for CYP3A and hydroxylations at the proline ring of the cyclopeptide moiety constituted a main metabolic pathway. Inhibitors and/or potent substrates for CYP3A4 might therefore inhibit the clearance of bromocriptine and lead to increased levels | :*In vitro studies using human liver microsomes showed that bromocriptine has a high affinity for CYP3A and hydroxylations at the proline ring of the cyclopeptide moiety constituted a main metabolic pathway. Inhibitors and/or potent substrates for CYP3A4 might therefore inhibit the clearance of bromocriptine and lead to increased levels. The participation of other major CYP enzymes such as 2D6, 2C8, and 2C19 on the metabolism of bromocriptine has not been evaluated. Bromocriptine is also an inhibitor of CYP3A4 with a calculated IC50 value of 1.69 μM. Given the low therapeutic concentrations of bromocriptine in patients (Cmax=0.82 nM), a significant alteration of the metabolism of a second drug whose clearance is mediated by CYP3A4 should not be expected. The potential effect of bromocriptine and its metabolites to act as inducers of CYP enzymes has not been reported. | ||
*Excretion | *Excretion | ||
| Line 531: | Line 370: | ||
<!--Nonclinical Toxicology--> | <!--Nonclinical Toxicology--> | ||
|nonClinToxic=*A 74-week study was conducted in mice using dietary levels of bromocriptine mesylate equivalent to oral doses of 10 and 50 mg/kg/day. A 100-week study in rats was conducted using dietary levels equivalent to oral doses of 1.7, 9.8, and 44 mg/kg/day. The highest doses tested in mice and rats were approximately 2.5 and 4.4 times, respectively, the maximum human dose administered in controlled clinical trials (100 mg/day) based on body surface area. Malignant uterine tumors, endometrial and myometrial were found in rats as follows: 0/50 control females, 2/50 females given 1.7 mg/kg daily, 7/49 females given 9.8 mg/kg daily, and 9/50 females given 44 mg/kg daily. The occurrence of these neoplasms is probably attributable to the high [[estrogen]]/[[progesterone]] ratio which occurs in rats as a result of the [[prolactin]]-inhibiting action of bromocriptine mesylate. The endocrine mechanisms believed to be involved in the rats are not present in humans. There is no known correlation between uterine malignancies occurring in bromocriptine-treated rats and human risk. In contrast to the findings in rats, the uteri from mice killed after 74 weeks of treatment did not exhibit evidence of drug-related changes. | |||
|nonClinToxic= | |||
*A 74-week study was conducted in mice using dietary levels of bromocriptine mesylate equivalent to oral doses of 10 and 50 mg/kg/day. A 100-week study in rats was conducted using dietary levels equivalent to oral doses of 1.7, 9.8, and 44 mg/kg/day. The highest doses tested in mice and rats were approximately 2.5 and 4.4 times, respectively, the maximum human dose administered in controlled clinical trials (100 mg/day) based on body surface area. Malignant uterine tumors, endometrial and myometrial were found in rats as follows: 0/50 control females, 2/50 females given 1.7 mg/kg daily, 7/49 females given 9.8 mg/kg daily, and 9/50 females given 44 mg/kg daily. The occurrence of these neoplasms is probably attributable to the high estrogen/progesterone ratio which occurs in rats as a result of the prolactin-inhibiting action of bromocriptine mesylate. The endocrine mechanisms believed to be involved in the rats are not present in humans. There is no known correlation between uterine malignancies occurring in bromocriptine-treated rats and human risk. In contrast to the findings in rats, the uteri from mice killed after 74 weeks of treatment did not exhibit evidence of drug-related changes. | |||
*Bromocriptine mesylate was evaluated for mutagenic potential in the battery of tests that included Ames bacterial mutation assay, mutagenic activity in vitro on V79 Chinese hamster fibroblasts, cytogenetic analysis of Chinese hamster bone marrow cells following in vivo treatment, and an in vivo micronucleus test for mutagenic potential in mice. | *Bromocriptine mesylate was evaluated for mutagenic potential in the battery of tests that included Ames bacterial mutation assay, mutagenic activity in vitro on V79 Chinese hamster fibroblasts, cytogenetic analysis of Chinese hamster bone marrow cells following in vivo treatment, and an in vivo micronucleus test for mutagenic potential in mice. | ||
| Line 540: | Line 376: | ||
*No mutagenic effects were obtained in any of these tests. | *No mutagenic effects were obtained in any of these tests. | ||
*Fertility and reproductive performance in female rats were not influenced adversely by treatment with bromocriptine beyond the predicted decrease in the weight of pups due to suppression of lactation. In males treated with 50 mg/kg of this drug, mating and fertility were within the normal range. Increased perinatal loss was produced in the subgroups of dams, sacrificed on day 21 postpartum (p.p.) after mating with males treated with the highest dose (50 mg/kg). | *Fertility and reproductive performance in female rats were not influenced adversely by treatment with bromocriptine beyond the predicted decrease in the weight of pups due to suppression of lactation. In males treated with 50 mg/kg of this drug, mating and fertility were within the normal range. Increased perinatal loss was produced in the subgroups of dams, sacrificed on day 21 [[postpartum]] (p.p.) after mating with males treated with the highest dose (50 mg/kg). | ||
<!--Clinical Studies--> | <!--Clinical Studies--> | ||
|clinicalStudies=*In about 75% of cases of [[amenorrhea]] and [[galactorrhea]], bromocriptine mesylate therapy suppresses the [[galactorrhea]] completely, or almost completely, and reinitiates normal ovulatory menstrual cycles. | |||
*Menses are usually reinitiated prior to complete suppression of [[galactorrhea]]; the time for this on average is 6-8 weeks. However, some patients respond within a few days, and others may take up to 8 months. | |||
* | *[[Galactorrhea]] may take longer to control depending on the degree of stimulation of the mammary tissue prior to therapy. At least a 75% reduction in secretion is usually observed after 8-12 weeks. Some patients may fail to respond even after 12 months of therapy. | ||
*In many acromegalic patients, bromocriptine mesylate produces a prompt and sustained reduction in circulating levels of serum [[growth hormone]]. | |||
*In many acromegalic patients, bromocriptine mesylate produces a prompt and sustained reduction in circulating levels of serum growth hormone. | |||
<!--How Supplied--> | <!--How Supplied--> | ||
|howSupplied=* Bromocriptine Mesylate Tablets, USP are available containing 2.5 mg of bromocriptine (as the mesylate). The 2.5 mg tablets are white to off-white, round, bevel edged snap tablets, debossed with "PAD" over "0106" on one side and scored on the other side. Complies with USP dissolution test 1. | |||
|howSupplied= | |||
* Bromocriptine Mesylate Tablets, USP are available containing 2.5 mg of bromocriptine (as the mesylate). The 2.5 mg tablets are white to off-white, round, bevel edged snap tablets, debossed with "PAD" over "0106" on one side and scored on the other side. Complies with USP dissolution test 1. | |||
:*Bottles of 30.........NDC 0574-0106-03 | :*Bottles of 30.........NDC 0574-0106-03 | ||
:*Bottles of 100.......NDC 0574-0106-01 | :*Bottles of 100.......NDC 0574-0106-01 | ||
| Line 567: | Line 397: | ||
<!--Patient Counseling Information--> | <!--Patient Counseling Information--> | ||
|fdaPatientInfo=*During clinical trials, [[dizziness]], drowsiness, faintness, fainting, and [[syncope]] have been reported early in the course of bromocriptine mesylate therapy. In postmarketing reports, bromocriptine mesylate has been associated with somnolence, and episodes of sudden sleep onset, particularly in patients with Parkinson’s disease. Sudden onset of sleep during daily activities, in some cases without awareness or warning signs, has been reported very rarely. All patients receiving bromocriptine mesylate should be cautioned with regard to engaging in activities requiring rapid and precise responses, such as driving an automobile or operating machinery. Patients being treated with bromocriptine mesylate and presenting with somnolence and/or sudden sleep episodes must be advised not to drive or engage in activities where impaired alertness may put themselves or others at risk of serious injury or death (e.g., operating machines). | |||
*Patients receiving bromocriptine mesylate for hyperprolactinemic states associated with [[macroadenoma]] or those who have had previous transsphenoidal surgery, should be told to report any persistent watery nasal discharge to their physician. Patients receiving bromocriptine mesylate for treatment of a macroadenoma should be told that discontinuation of drug may be associated with rapid regrowth of the [[tumor]] and recurrence of their original symptoms. | |||
*Patients receiving bromocriptine mesylate for hyperprolactinemic states associated with macroadenoma or those who have had previous transsphenoidal surgery, should be told to report any persistent watery nasal discharge to their physician. Patients receiving bromocriptine mesylate for treatment of a macroadenoma should be told that discontinuation of drug may be associated with rapid regrowth of the tumor and recurrence of their original symptoms. | |||
*Patients and their caregivers should be alerted to the possibility that they may experience intense urges to spend money uncontrollably, intense urges to gamble, increased sexual urges and other intense urges and the inability to control these urges while taking bromocriptine mesylate | *Patients and their caregivers should be alerted to the possibility that they may experience intense urges to spend money uncontrollably, intense urges to gamble, increased sexual urges and other intense urges and the inability to control these urges while taking bromocriptine mesylate. | ||
*Especially during the first days of treatment, hypotensive reactions may occasionally occur and result in reduced alertness, particular care should be exercised when driving a vehicle or operating machinery. | *Especially during the first days of treatment, [[hypotensive]] reactions may occasionally occur and result in reduced alertness, particular care should be exercised when driving a vehicle or operating machinery. | ||
<!--Precautions with Alcohol--> | <!--Precautions with Alcohol--> | ||
|alcohol=* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |||
|alcohol= | |||
* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |||
<!--Brand Names--> | <!--Brand Names--> | ||
|brandNames=* BROMOCRIPTINE MESYLATE®<ref>{{Cite web | title = BROMOCRIPTINE MESYLATE- bromocriptine mesylate tablet | url = http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0ee2da00-faf8-4c51-a696-9462fa4f6050 }}</ref> | |||
|brandNames= | |||
* BROMOCRIPTINE MESYLATE®<ref>{{Cite web | title = BROMOCRIPTINE MESYLATE- bromocriptine mesylate tablet | url = http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0ee2da00-faf8-4c51-a696-9462fa4f6050 }}</ref> | |||
<!--Look-Alike Drug Names--> | <!--Look-Alike Drug Names--> | ||
|lookAlike=<!--Drug Shortage Status--> | |||
|lookAlike= | |||
<!--Drug Shortage Status--> | |||
|drugShortage= | |drugShortage= | ||
}} | }} | ||
<!--Pill Image--> | <!--Pill Image--> | ||
{{PillImage|fileName=Parlodel_NDC_00780017.jpg|drugName=Parlodel|NDC=00780017|drugAuthor=Novartis Pharmaceuticals Corporation|ingredients=BROMOCRIPTINE MESYLATE[BROMOCRIPTINE]|pillImprint=PARLODEL;2;12|dosageValue=2.5|dosageUnit=mg|pillColor=White|pillShape=Round|pillSize=8|pillScore=2}} | |||
{{PillImage|fileName=Parlodel_NDC_00780102.jpg|drugName=Parlodel|NDC=00780102|drugAuthor=Novartis Pharmaceuticals Corporation|ingredients=BROMOCRIPTINE MESYLATE[BROMOCRIPTINE]|pillImprint=PARLODEL;5;mg;S|dosageValue=5|dosageUnit=mg|pillColor=Brown|pillShape=Capsule|pillSize=16|pillScore=1}} | |||
{{PillImage|fileName=Bromocriptine_Mesylate_NDC_03782042.jpg|drugName=Bromocriptine Mesylate|NDC=03782042|drugAuthor=Mylan Pharmaceuticals Inc.|ingredients=BROMOCRIPTINE MESYLATE[BROMOCRIPTINE]|pillImprint=M;42|dosageValue=2.5|dosageUnit=mg|pillColor=White|pillShape=Round|pillSize=7|pillScore=2}} | |||
{{PillImage|fileName=Bromocriptine_Mesylate_NDC_03787096.jpg|drugName=Bromocriptine Mesylate|NDC=03787096|drugAuthor=Mylan Pharmaceuticals Inc.|ingredients=BROMOCRIPTINE MESYLATE[BROMOCRIPTINE]|pillImprint=MYLAN;7096|dosageValue=5|dosageUnit=mg|pillColor=Brown;White|pillShape=Capsule|pillSize=14|pillScore=1}} | |||
{{PillImage|fileName=Bromocriptine_mesylate_NDC_05740106.jpg|drugName=Bromocriptine mesylate|NDC=05740106|drugAuthor=Paddock Laboratories, Inc.|ingredients=Bromocriptine mesylate[Bromocriptine]|pillImprint=PAD;0106|dosageValue=2.5|dosageUnit=mg|pillColor=White|pillShape=Round|pillSize=8|pillScore=2}} | |||
{{PillImage|fileName=CYCLOSET_NDC_680120258.jpg|drugName=CYCLOSET|NDC=680120258|drugAuthor=Santarus, Inc.|ingredients=bromocriptine mesylate[bromocriptine]|pillImprint=C;9|dosageValue=0.8|dosageUnit=mg|pillColor=White|pillShape=Round|pillSize=6|pillScore=1}} | |||
<!--Label Display Image--> | <!--Label Display Image--> | ||
{{LabelImage | {{LabelImage | ||
|fileName={{PAGENAME}} | |fileName={{PAGENAME}}02.png|This image is provided by the National Library of Medicine. | ||
}} | |||
{{LabelImage | |||
|fileName={{PAGENAME}}03.png|This image is provided by the National Library of Medicine. | |||
}} | }} | ||
{{LabelImage | {{LabelImage | ||
|fileName={{PAGENAME}} | |fileName={{PAGENAME}}04.png|This image is provided by the National Library of Medicine. | ||
}} | }} | ||
Latest revision as of 18:19, 18 August 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Vignesh Ponnusamy, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Bromocriptine is a dopamine agonist that is FDA approved for the treatment of acromegaly, hyperprolactinemia, and parkinson's disease. Common adverse reactions include hypotension, constipation, diarrhea, indigestion, nausea, vomiting, asthenia, dizziness, headache, somnolence, amblyopia, rhinitis, sinusitis, and fatigue.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Hyperprolactinemic States
- The initial dosage of bromocriptine mesylate tablets in adults is ½ to one 2.5 mg scored tablet daily. An additional 2.5 mg tablet may be added to the treatment regimen as tolerated every 2-7 days until an optimal therapeutic response is achieved. The therapeutic dosage ranged from 2.5-15 mg daily in adults studied clinically.
- In order to reduce the likelihood of prolonged exposure to bromocriptine mesylate tablets should an unsuspected pregnancy occur, a mechanical contraceptive should be used in conjunction with bromocriptine mesylate tablets therapy until normal ovulatory menstrual cycles have been restored. Contraception may then be discontinued in patients desiring pregnancy.
- Thereafter, if menstruation does not occur within 3 days of the expected date, bromocriptine mesylate therapy should be discontinued and a pregnancy test performed.
Acromegaly
- Virtually all acromegalic patients receiving therapeutic benefit from bromocriptine mesylate tablets also have reductions in circulating levels of growth hormone. Therefore, periodic assessment of circulating levels of growth hormone will, in most cases, serve as a guide in determining the therapeutic potential of bromocriptine mesylate tablets. If, after a brief trial with bromocriptine mesylate therapy, no significant reduction in growth hormone levels has taken place, careful assessment of the clinical features of the disease should be made, and if no change has occurred, dosage adjustment or discontinuation of therapy should be considered.
- The initial recommended dosage is ½ to one 2.5 mg bromocriptine mesylate tablet on retiring (with food) for 3 days. An additional ½ to 1 tablet should be added to the treatment regimen as tolerated every 3-7 days until the patient obtains optimal therapeutic benefit. Patients should be reevaluated monthly and the dosage adjusted based on reductions of growth hormone or clinical response. The usual optimal therapeutic dosage range of bromocriptine mesylate tablets varies from 20-30 mg/day in most patients. The maximal dosage should not exceed 100 mg/day.
- Patients treated with pituitary irradiation should be withdrawn from bromocriptine mesylate tablets therapy on a yearly basis to assess both the clinical effects of radiation on the disease process as well as the effects of bromocriptine mesylate tablets therapy. Usually a 4-8 week withdrawal period is adequate for this purpose. Recurrence of the signs/symptoms or increases in growth hormone indicate the disease process is still active and further courses of bromocriptine mesylate tablets should be considered.
Parkinson's Disease
- The basic principle of bromocriptine mesylate tablets therapy is to initiate treatment at a low dosage and, on an individual basis, increase the daily dosage slowly until a maximum therapeutic response is achieved. The dosage of levodopa during this introductory period should be maintained, if possible. The initial dose of bromocriptine mesylate is ½ of a 2.5 mg tablet twice daily with meals. Assessments are advised at 2-week intervals during dosage titration to ensure that the lowest dosage producing an optimal therapeutic response is not exceeded. If necessary, the dosage may be increased every 14-28 days by 2½ mg/day with meals. Should it be advisable to reduce the dosage of levodopa because of adverse reactions, the daily dosage of bromocriptine mesylate, if increased, should be accomplished gradually in small (2½ mg) increments.
- The safety of bromocriptine mesylate has not been demonstrated in dosages exceeding 100 mg/day.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Bromocriptine in adult patients.
Non–Guideline-Supported Use
Female infertility - In vitro fertilization
- Bromocriptine initially at 1.25 milligrams daily (mg/day) between days 4 to 6 of the follicular phase, then at 2.5 mg/day until 3 days after onset of menstruation.[1]
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Hyperprolactinemic States
- Based on limited data in children of age 11 to 15, the initial dose is ½ to one 2.5 mg scored tablet daily. Dosing may need to be increased as tolerated until a therapeutic response is achieved. The therapeutic dosage ranged from 2.5-10 mg daily in children with prolactin-secreting pituitary adenomas.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Bromocriptine in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Bromocriptine in pediatric patients.
Contraindications
- Hypersensitivity to bromocriptine or to any of the excipients of bromocriptine mesylate tablets, uncontrolled hypertension and sensitivity to any ergot alkaloids. In patients being treated for hyperprolactinemia, bromocriptine mesylate should be withdrawn when pregnancy is diagnosed. In the event that bromocriptine mesylate is reinstituted to control a rapidly expanding macroadenoma and a patient experiences a hypertensive disorder of pregnancy, the benefit of continuing bromocriptine mesylate must be weighed against the possible risk of its use during a hypertensive disorder of pregnancy.
- When bromocriptine mesylate is being used to treat acromegaly, prolactinoma, or Parkinson’s disease in patients who subsequently become pregnant, a decision should be made as to whether the therapy continues to be medically necessary or can be withdrawn. If it is continued, the drug should be withdrawn in those who may experience hypertensive disorders of pregnancy (including eclampsia, preeclampsia, or pregnancy-induced hypertension) unless withdrawal of bromocriptine mesylate is considered to be medically contraindicated.
- The drug should not be used during the postpartum period in women with a history of coronary artery disease and other severe cardiovascular conditions unless withdrawal is considered medically contraindicated. If the drug is used in the postpartum period, the patient should be observed with caution.
Warnings
- Since hyperprolactinemia with amenorrhea/galactorrhea and infertility has been found in patients with pituitary tumors, a complete evaluation of the pituitary is indicated before treatment with bromocriptine mesylate.
- If pregnancy occurs during bromocriptine mesylate administration, careful observation of these patients is mandatory. Prolactin-secreting adenomas may expand and compression of the optic or other cranial nerves may occur, emergency pituitary surgery becoming necessary. In most cases, the compression resolves following delivery. Reinitiation of bromocriptine mesylate treatment has been reported to produce improvement in the visual fields of patients in whom nerve compression has occurred during pregnancy. The safety of bromocriptine mesylate treatment during pregnancy to the mother and fetus has not been established.
- Bromocriptine mesylate has been associated with somnolence, and episodes of sudden sleep onset, particularly in patients with Parkinson’s disease. Sudden onset of sleep during daily activities, in some cases without awareness or warning signs, has been reported. Patients must be informed of this and advised not to drive or operate machines during treatment with bromocriptine. Patients who have experienced somnolence and/or an episode of sudden sleep onset must not drive or operate machines. Furthermore, a reduction of dosage or termination of therapy may be considered.
- Symptomatic hypotension can occur in patients treated with bromocriptine mesylate for any indication. In postpartum studies with bromocriptine mesylate, decreases in supine systolic and diastolic pressures of greater than 20 mm and 10 mm Hg, respectively, have been observed in almost 30% of patients receiving bromocriptine mesylate. On occasion, the drop in supine systolic pressure was as much as 50-59 mm of Hg.
- Since, especially during the first days of treatment, hypotensive reactions may occasionally occur and result in reduced alertness, particular care should be exercised when driving a vehicle or operating machinery.
- While hypotension during the start of therapy with bromocriptine mesylate occurs in some patients, in rare cases serious adverse events, including hypertension, myocardial infarction, seizures, stroke, have been reported in postpartum women treated with bromocriptine mesylate. Hypertension has been reported, sometimes at the initiation of therapy, but often developing in the second week of therapy; seizures have also been reported both with and without the prior development of hypertension; stroke has been reported mostly in postpartum patients whose prenatal and obstetric courses had been uncomplicated. Many of these patients experiencing seizures (including cases of status epilepticus) and/or strokes reported developing a constant and often progressively severe headache hours to days prior to the acute event. Some cases of strokes and seizures were also preceded by visual disturbances (blurred vision, and transient cortical blindness). Cases of acute myocardial infarction have also been reported.
- Although a causal relationship between bromocriptine mesylate administration and hypertension, seizures, strokes, and myocardial infarction in postpartum women has not been established, use of the drug in patients with uncontrolled hypertension is not recommended. In patients being treated for hyperprolactinemia, bromocriptine mesylate should be withdrawn when pregnancy is diagnosed. In the event that bromocriptine mesylate is reinstituted to control a rapidly expanding macroadenoma and a patient experiences a hypertensive disorder of pregnancy, the benefit of continuing bromocriptine mesylate must be weighed against the possible risk of its use during a hypertensive disorder of pregnancy. When bromocriptine mesylate is being used to treat acromegaly or Parkinson’s disease in patients who subsequently become pregnant, a decision should be made as to whether the therapy continues to be medically necessary or can be withdrawn. If it is continued, the drug should be withdrawn in those who may experience hypertensive disorders of pregnancy (including eclampsia, preeclampsia, or pregnancy-induced hypertension) unless withdrawal of bromocriptine mesylate is considered to be medically contraindicated. Because of the possibility of an interaction between bromocriptine mesylate and other ergot alkaloids, the concomitant use of these medications is not recommended. Periodic monitoring of the blood pressure, particularly during the first weeks of therapy is prudent. If hypertension, severe, progressive, or unremitting headache (with or without visual disturbance), or evidence of CNS toxicity develops, drug therapy should be discontinued and the patient should be evaluated promptly. Particular attention should be paid to patients who have recently been treated or are on concomitant therapy with drugs that can alter blood pressure. Their concomitant use in the puerperium is not recommended.
- Among patients on bromocriptine mesylate, particularly on long-term and high-dose treatment, pleural and pericardial effusions, as well as pleural and pulmonary fibrosis and constrictive pericarditis, have been reported. Patients with unexplained pleuropulmonary disorders should be examined thoroughly and discontinuation of bromocriptine mesylate therapy should be considered. In those instances in which bromocriptine mesylate treatment was terminated, the changes slowly reverted towards normal.
- In a few patients on bromocriptine mesylate, particularly on long-term and high-dose treatment, retroperitoneal fibrosis has been reported. To ensure recognition of retroperitoneal fibrosis at an early reversible stage it is recommended that its manifestations (e.g., back pain, edema of the lower limbs, impaired kidney function) should be watched in this category of patients. Bromocriptine mesylate medication should be withdrawn if fibrotic changes in the retroperitoneum are diagnosed or suspected.
Precautions
- General
- Safety and efficacy of bromocriptine mesylate have not been established in patients with renal or hepatic disease. Care should be exercised when administering bromocriptine mesylate therapy concomitantly with other medications known to lower blood pressure.
- The drug should be used with caution in patients with a history of psychosis or cardiovascular disease. If acromegalic patients or patients with prolactinoma or Parkinson's disease are being treated with bromocriptine mesylate during pregnancy, they should be cautiously observed, particularly during the postpartum period if they have a history of cardiovascular disease.
- Patients with rare hereditary problems of galactose intolerance, severe lactase deficiency or glucose-galactose malabsorption should not take this medicine.
- Hyperprolactinemic States
- Visual field impairment is a known complication of macroprolactinoma. Effective treatment with bromocriptine mesylate leads to a reduction in hyperprolactinemia and often to a resolution of the visual impairment. In some patients, however, a secondary deterioration of visual fields may subsequently develop despite normalized prolactin levels and tumor shrinkage, which may result from traction on the optic chiasm which is pulled down into the now partially empty sella. In these cases, the visual field defect may improve on reduction of bromocriptine dosage while there is some elevation of prolactin and some tumor re-expansion. Monitoring of visual fields in patients with macroprolactinoma is therefore recommended for an early recognition of secondary field loss due to chiasmal herniation and adaptation of drug dosage.
- The relative efficacy of bromocriptine mesylate versus surgery in preserving visual fields is not known. Patients with rapidly progressive visual field loss should be evaluated by a neurosurgeon to help decide on the most appropriate therapy.
- Since pregnancy is often the therapeutic objective in many hyperprolactinemic patients presenting with amenorrhea/galactorrhea and hypogonadism (infertility), a careful assessment of the pituitary is essential to detect the presence of a prolactin-secreting adenoma. Patients not seeking pregnancy, or those harboring large adenomas, should be advised to use contraceptive measures, other than oral contraceptives, during treatment with bromocriptine mesylate. Since pregnancy may occur prior to reinitiation of menses, a pregnancy test is recommended at least every 4 weeks during the amenorrheic period, and, once menses are reinitiated, every time a patient misses a menstrual period. Treatment with bromocriptine mesylate should be discontinued as soon as pregnancy has been established. Patients must be monitored closely throughout pregnancy for signs and symptoms that may signal the enlargement of a previously undetected or existing prolactin-secreting tumor. Discontinuation of bromocriptine mesylate treatment in patients with known macroadenomas has been associated with rapid regrowth of tumor and increase in serum prolactin in most cases.
- Cerebrospinal fluid rhinorrhea has been observed in some patients with prolactin-secreting adenomas treated with bromocriptine mesylate.
- Acromegaly
- Cld-sensitive digital vasospasm has been observed in some acromegalic patients treated with bromocriptine mesylate. The response, should it occur, can be reversed by reducing the dose of bromocriptine mesylate and may be prevented by keeping the fingers warm. Cases of severe gastrointestinal bleeding from peptic ulcers have been reported, some fatal. Although there is no evidence that bromocriptine mesylate increases the incidence of peptic ulcers in acromegalic patients, symptoms suggestive of peptic ulcer should be investigated thoroughly and treated appropriately. Patients with a history of peptic ulcer or gastrointestinal bleeding should be observed carefully during treatment with bromocriptine mesylate.
- Possible tumor expansion while receiving bromocriptine mesylate therapy has been reported in a few patients. Since the natural history of growth hormone-secreting tumors is unknown, all patients should be carefully monitored and, if evidence of tumor expansion develops, discontinuation of treatment and alternative procedures considered.
- Parkinson's Disease
- Safety during long-term use for more than 2 years at the doses required for parkinsonism has not been established.
- As with any chronic therapy, periodic evaluation of hepatic, hematopoietic, cardiovascular, and renal function is recommended. Symptomatic hypotension can occur and, therefore, caution should be exercised when treating patients receiving antihypertensive drugs.
- High doses of bromocriptine mesylate may be associated with confusion and mental disturbances. Since parkinsonian patients may manifest mild degrees of dementia, caution should be used when treating such patients.
- Bromocriptine mesylate administered alone or concomitantly with levodopa may cause hallucinations (visual or auditory). Hallucinations usually resolve with dosage reduction; occasionally, discontinuation of bromocriptine mesylate is required. Rarely, after high doses, hallucinations have persisted for several weeks following discontinuation of bromocriptine mesylate.
- Postmarketing reports suggest that patients treated with anti-Parkinson medications can experience intense urges to gamble, increased sexual urges, intense urges to spend money uncontrollably, and other intense urges. Patients may be unable to control these urges while taking one or more of the medications that are generally used for the treatment of Parkinson’s disease and that increase central dopaminergic tone, including bromocriptine mesylate. In some cases, although not all, these urges were reported to have stopped when the dose was reduced or the medication was discontinued. Because patients may not recognize these behaviors as abnormal it is important for prescribers to specifically ask patients or their caregivers about the development of new or increased gambling urges, sexual urges, uncontrolled spending or other urges while being treated with bromocriptine mesylate. Physicians should consider dose reduction or stopping the medication if a patient develops such urges while taking bromocriptine mesylate.
- As with levodopa, caution should be exercised when administering bromocriptine mesylate to patients with a history of myocardial infarction who have a residual atrial, nodal, or ventricular arrhythmia.
- Retroperitoneal fibrosis has been reported in a few patients receiving long-term therapy (2-10 years) with bromocriptine mesylate in doses ranging from 30-140 mg daily.
- Epidemiological studies have shown that patients with Parkinson’s disease have a higher risk (2-approximately 6-fold higher) of developing melanoma than the general population. Whether the increased risk observed was due to Parkinson’s disease or other factors, such as drugs used to treat Parkinson’s disease, is unclear. For the reasons stated above, patients and providers are advised to monitor for melanomas frequently and on a regular basis when using bromocriptine mesylate for any indication. Ideally, periodic skin examinations should be performed by appropriately qualified individuals (e.g. dermatologists).
- Discontinuation of bromocriptine mesylate should be undertaken gradually whenever possible, even if the patient is to remain on L-dopa. A symptom complex resembling the neuroleptic malignant syndrome (characterized by elevated temperature, muscular rigidity, altered consciousness, and autonomic instability), with no other obvious etiology, has been reported in association with rapid dose reduction, withdrawal of, or changes in antiparkinsonian therapy.
Adverse Reactions
Clinical Trials Experience
Hyperprolactinemic Indications
- The incidence of adverse effects is quite high (69%) but these are generally mild to moderate in degree. Therapy was discontinued in approximately 5% of patients because of adverse effects. These in decreasing order of frequency are: nausea (49%), headache (19%), dizziness (17%), fatigue (7%), lightheadedness (5%), vomiting (5%), abdominal cramps (4%), nasal congestion (3%), constipation (3%), diarrhea (3%) and drowsiness (3%).
- A slight hypotensive effect may accompany bromocriptine mesylate treatment. The occurrence of adverse reactions may be lessened by temporarily reducing dosage to ½ tablet 2 or 3 times daily. A few cases of cerebrospinal fluid rhinorrhea have been reported in patients receiving bromocriptine mesylate for treatment of large prolactinomas. This has occurred rarely, usually only in patients who have received previous transsphenoidal surgery, pituitary radiation, or both, and who were receiving bromocriptine mesylate for tumor recurrence. It may also occur in previously untreated patients whose tumor extends into the sphenoid sinus.
Acromegaly
- The most frequent adverse reactions encountered in acromegalic patients treated with bromocriptine mesylate were: nausea (18%), constipation (14%), postural/orthostatic hypotension (6%), anorexia (4%), dry mouth/nasal stuffiness (4%), indigestion/dyspepsia (4%), digital vasospasm (3%), drowsiness/tiredness (3%) and vomiting (2%).
- Less frequent adverse reactions (less than 2%) were: gastrointestinal bleeding, dizziness, exacerbation of Raynaud’s syndrome, headache and syncope. Rarely (less than 1%) hair loss, alcohol potentiation, faintness, lightheadedness, arrhythmia, ventricular tachycardia, decreased sleep requirement, visual hallucinations, lassitude, shortness of breath, bradycardia, vertigo, paresthesia, sluggishness, vasovagal attack, delusional psychosis, paranoia, insomnia, heavy headedness, reduced tolerance to cold, tingling of ears, facial pallor and muscle cramps have been reported.
Parkinson's Disease
- In clinical trials in which bromocriptine mesylate was administered with concomitant reduction in the dose of levodopa/carbidopa, the most common newly appearing adverse reactions were: nausea, abnormal involuntary movements, hallucinations, confusion, “on-off’’ phenomenon, dizziness, drowsiness, faintness/fainting, vomiting, asthenia, abdominal discomfort, visual disturbance, ataxia, insomnia, depression, hypotension, shortness of breath, constipation, and vertigo.
- Less common adverse reactions which may be encountered include: anorexia, anxiety, blepharospasm, dry mouth, dysphagia, edema of the feet and ankles, erythromelalgia, epileptiform seizure, fatigue, headache, lethargy, mottling of skin, nasal stuffiness, nervousness, nightmares, paresthesia, skin rash, urinary frequency, urinary incontinence, urinary retention, and rarely, signs and symptoms of ergotism such as tingling of fingers, cold feet, numbness, muscle cramps of feet and legs or exacerbation of Raynaud’s Syndrome.
- Abnormalities in laboratory tests may include elevations in blood urea nitrogen, SGOT, SGPT, GGPT, CPK, alkaline phosphatase and uric acid, which are usually transient and not of clinical significance.
Postpartum Patients
- In postpartum studies with bromocriptine mesylate, 23 percent of postpartum patients treated had at least 1 side effect, but they were generally mild to moderate in degree. Therapy was discontinued in approximately 3% of patients. The most frequently occurring adverse reactions were: headache (10%), dizziness (8%), nausea (7%), vomiting (3%), fatigue (1.0%), syncope (0.7%), diarrhea (0.4%) and cramps (0.4%). Decreases in blood pressure (≥ 20 mm Hg systolic and ≥ 10 mm Hg diastolic) occurred in 28% of patients at least once during the first 3 postpartum days; these were usually of a transient nature. Reports of fainting in the puerperium may possibly be related to this effect. In postmarketing experience in the U.S., serious adverse reactions reported include 72 cases of seizures (including 4 cases of status epilepticus), 30 cases of stroke, and 9 cases of myocardial infarction among postpartum patients. Seizure cases were not necessarily accompanied by the development of hypertension. An unremitting and often progressively severe headache, sometimes accompanied by visual disturbance, often preceded by hours to days many cases of seizure and/or stroke. Most patients had shown no evidence of any of the hypertensive disorders of pregnancy including eclampsia, preeclampsia or pregnancy-induced hypertension. One stroke case was associated with sagittal sinus thrombosis, and another was associated with cerebral and cerebellar vasculitis. One case of myocardial infarction was associated with unexplained disseminated intravascular coagulation and a second occurred in conjunction with use of another ergot alkaloid. The relationship of these adverse reactions to bromocriptine mesylate administration has not been established.
- In rare cases serious adverse events, including hypertension, myocardial infarction, seizures, stroke, or psychic disorders have been reported in postpartum women treated with bromocriptine mesylate. In some patients the development of seizures or stroke was preceded by severe headache and/or transient visual disturbances. Although the causal relationship of these events to the drug is uncertain, periodic monitoring of blood pressure is advisable in postpartum women receiving bromocriptine mesylate. If hypertension, severe, progressive, or unremitting headache (with or without visual disturbances), or evidence of CNS toxicity develop, the administration of bromocriptine mesylate should be discontinued and the patient should be evaluated promptly.
- Particular caution is required in patients who have recently been treated or are on concomitant therapy with drugs that can alter blood pressure, e.g. vasoconstrictors such as sympathomimetics or ergot alkaloids including ergometrine or methylergometrine and their concomitant use in the puerperium is not recommended.
Postmarketing Experience
- The following adverse reactions have been reported during postapproval use of bromocriptine mesylate (All Indications Combined). Because adverse reactions from spontaneous reports are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Psychiatric disorders
Confusion, psychomotor agitation/excitation, hallucinations, psychotic disorders, insomnia, libido increase, hypersexuality.
Nervous system disorders
Headache, drowsiness, dizziness, dyskinaesia, somnolence, paraesthesia, excess daytime somnolence, sudden onset of sleep.
Eye disorders
Visual disturbance, blurred vision.
Ear and labyrinth disorders
Cardiac disorders
Pericardial effusion, constrictive pericarditis, tachycardia, bradycardia, arrhythmia, cardiac valve fibrosis.
Vascular disorders
Hypotension, orthostatic hypotension (very rarely leading to syncope), reversible pallor of fingers and toes induced by cold (especially in patients with history of Raynaud's phenomenon)
Respiratory, thoracic and mediastinal disorders
Nasal congestion, pleural effusion, pleural fibrosis, pleurisy, pulmonary fibrosis, dyspnea.
Gastrointestinal disorders
Nausea, constipation, vomiting, dry mouth, diarrhea, abdominal pain, retroperitoneal fibrosis, gastrointestinal ulcer, gastrointestinal haemorrhage.
Skin and subcutaneous tissue disorders
Allergic skin reactions, hair loss.
Musculoskeletal and connective tissue disorders
Leg cramps.
General disorders and administration site conditions
Fatigue, peripheral edema, a syndrome resembling Neuroleptic Malignant Syndrome on abrupt withdrawal of bromocriptine mesylate.
Drug Interactions
- The risk of using bromocriptine mesylate in combination with other drugs has not been systematically evaluated, but alcohol may potentiate the side effects of bromocriptine mesylate. Bromocriptine mesylate may interact with dopamine antagonists, butyrophenones, and certain other agents. Compounds in these categories result in a decreased efficacy of bromocriptine mesylate: phenothiazines, haloperidol, metoclopramide, pimozide. Bromocriptine is a substrate of CYP3A4. Caution should therefore be used when co-administering drugs which are strong inhibitors of this enzyme (such as azole antimycotics, HIV protease inhibitors). The concomitant use of macrolide antibiotics such as erythromycin was shown to increase the plasma levels of bromocriptine (mean AUC and Cmax values increased 3.7-fold and 4.6-fold, respectively). 1The concomitant treatment of acromegalic patients with bromocriptine and octreotide led to increased plasma levels of bromocriptine (bromocriptine AUC increased about 38%). 4Concomitant use of bromocriptine mesylate with other ergot alkaloids is not recommended. Dose adjustment may be necessary in those cases where high doses of bromocriptine are being used (such as Parkinson’s disease indication).
Use in Specific Populations
Pregnancy
- Pregnancy Category B
- Administration of 10-30 mg/kg of bromocriptine to 2 strains of rats on days 6-15 postcoitum (p.c.) as well as a single dose of 10 mg/kg on day 5 p.c. interfered with nidation.
- Three mg/kg given on days 6-15 were without effect on nidation, and did not produce any anomalies. In animals treated from day 8-15 p.c., i.e., after implantation, 30 mg/kg produced increased prenatal mortality in the form of increased incidence of embryonic resorption. One anomaly, aplasia of spinal vertebrae and ribs, was found in the group of 262 fetuses derived from the dams treated with 30 mg/kg bromocriptine. No fetotoxic effects were found in offspring of dams treated during the peri- or postnatal period.
- Two studies were conducted in rabbits (2 strains) to determine the potential to interfere with nidation. Dose levels of 100 or 300 mg/kg/day from day 1 to day 6 p.c. did not adversely affect nidation. The high dose was approximately 63 times the maximum human dose administered in controlled clinical trials (100 mg/day), based on body surface area. In New Zealand white rabbits, some embryo mortality occurred at 300 mg/kg which was a reflection of overt maternal toxicity. Three studies were conducted in 2 strains of rabbits to determine the teratological potential of bromocriptine at dose levels of 3, 10, 30, 100, and 300 mg/kg given from day 6 to day 18 p.c. In 2 studies with the Yellow-silver strain, cleft palate was found in 3 and 2 fetuses at maternally toxic doses of 100 and 300 mg/kg, respectively. One control fetus also exhibited this anomaly. In the third study conducted with New Zealand white rabbits using an identical protocol, no cleft palates were produced.
- No teratological or embryotoxic effects of bromocriptine were produced in any of 6 offspring from 6 monkeys at a dose level of 2 mg/kg.
- Information concerning 1276 pregnancies in women taking bromocriptine mesylate has been collected. In the majority of cases, bromocriptine mesylate was discontinued within 8 weeks into pregnancy (mean 28.7 days), however, 8 patients received the drug continuously throughout pregnancy. The mean daily dose for all patients was 5.8 mg (range 1-40 mg).
- Of these 1276 pregnancies, there were 1088 full-term deliveries (4 stillborn), 145 spontaneous abortions (11.4%), and 28 induced abortions (2.2%). Moreover, 12 extrauterine gravidities and 3 hydatidiform moles (twice in the same patient) caused early termination of pregnancy. These data compare favorably with the abortion rate (11%-25%) cited for pregnancies induced by clomiphene citrate, menopausal gonadotropin, and chorionic gonadotropin.
- Although spontaneous abortions often go unreported, especially prior to 20 weeks of gestation, their frequency has been estimated to be 15%.
- The incidence of birth defects in the population at large ranges from 2%-4.5%. The incidence in 1109 live births from patients receiving bromocriptine is 3.3%.
- There is no suggestion that bromocriptine mesylate contributed to the type or incidence of birth defects in this group of infants.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Bromocriptine in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Bromocriptine during labor and delivery.
Nursing Mothers
- Bromocriptine mesylate should not be used during lactation in postpartum women.
Pediatric Use
- The safety and effectiveness of bromocriptine for the treatment of prolactin-secreting pituitary adenomas have been established in patients age 16 to adult. No data are available for bromocriptine use in pediatric patients under the age of 8 years. A single 8-year-old patient treated with bromocriptine for a prolactin-secreting pituitary macroadenoma has been reported without therapeutic response.
- The use of bromocriptine for the treatment of prolactin-secreting adenomas in pediatric patients in the age group 11 to under 16 years is supported by evidence from well-controlled trials in adults, with additional data in a limited number (n=14) of children and adolescents 11 to 15 years of age with prolactin-secreting pituitary macro- and microadenomas who have been treated with bromocriptine. Of the 14 reported patients, 9 had successful outcomes, 3 partial responses, and 2 failed to respond to bromocriptine treatment. Chronic hypopituitarism complicated macroadenoma treatment in 5 of the responders, both in patients receiving bromocriptine alone and in those who received bromocriptine in combination with surgical treatment and/or pituitary irradiation.
- Safety and effectiveness of bromocriptine in pediatric patients have not been established for any other indication listed in the INDICATIONS AND USAGE section.
Geriatic Use
- Clinical studies for bromocriptine mesylate did not include sufficient numbers of subjects aged 65 and over to determine whether the elderly respond differently from younger subjects. However, other reported clinical experiences, including postmarketing reporting of adverse events, have not identified differences in response or tolerability between elderly and younger patients. Even though no variation in efficacy or adverse reaction profile in geriatric patients taking bromocriptine mesylate has been observed, greater sensitivity of some elderly individuals cannot be categorically ruled out. In general, dose selection for an elderly patient should be cautious, starting at the lower end of the dose range, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy in this population.
Gender
There is no FDA guidance on the use of Bromocriptine with respect to specific gender populations.
Race
There is no FDA guidance on the use of Bromocriptine with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Bromocriptine in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Bromocriptine in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Bromocriptine in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Bromocriptine in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
There is limited information regarding Monitoring of Bromocriptine in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Bromocriptine in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- The most commonly reported signs and symptoms associated with acute bromocriptine mesylate overdose are: nausea, vomiting, constipation, diaphoresis, dizziness, pallor, severe hypotension, malaise, confusion, lethargy, drowsiness, delusions, hallucinations, and repetitive yawning. The lethal dose has not been established and the drug has a very wide margin of safety. However, one death occurred in a patient who committed suicide with an unknown quantity of bromocriptine mesylate and chloroquine.
Management
- Treatment of overdose consists of removal of the drug by emesis (if conscious), gastric lavage, activated charcoal, or saline catharsis. Careful supervision and recording of fluid intake and output is essential. Hypotension should be treated by placing the patient in the Trendelenburg position and administering I.V. fluids. If satisfactory relief of hypotension cannot be achieved by using the above measures to their fullest extent, vasopressors should be considered.
Chronic Overdose
There is limited information regarding Chronic Overdose of Bromocriptine in the drug label.
Pharmacology
Mechanism of Action
- Bromocriptine mesylate is a dopamine receptor agonist, which activates post-synaptic dopamine receptors. The dopaminergic neurons in the tuberoinfundibular process modulate the secretion of prolactin from the anterior pituitary by secreting a prolactin inhibitory factor (thought to be dopamine); in the corpus striatum the dopaminergic neurons are involved in the control of motor function. Clinically, bromocriptine mesylate significantly reduces plasma levels of prolactin in patients with physiologically elevated prolactin as well as in patients with hyperprolactinemia. The inhibition of physiological lactation as well as galactorrhea in pathological hyperprolactinemic states is obtained at dose levels that do not affect secretion of other tropic hormones from the anterior pituitary. Experiments have demonstrated that bromocriptine induces long-lasting stereotyped behavior in rodents and turning behavior in rats having unilateral lesions in the substantia nigra. These actions, characteristic of those produced by dopamine, are inhibited by dopamine antagonists and suggest a direct action of bromocriptine on striatal dopamine receptors.
- Bromocriptine mesylate is a nonhormonal, nonestrogenic agent that inhibits the secretion of prolactin in humans, with little or no effect on other pituitary hormones, except in patients with acromegaly, where it lowers elevated blood levels of growth hormone in the majority of patients.
Structure
- Bromocriptine mesylate is an ergot derivative with potent dopamine receptor agonist activity. Each bromocriptine mesylate tablet, USP for oral administration contains 2.5 mg bromocriptine (as the mesylate). Bromocriptine mesylate is chemically designated as Ergotaman-3',6',18-trione, 2-bromo-12'-hydroxy-2'-(1-methylethyl)-5'-(2-methylpropyl)-,(5'α)-monomethanesulfonate (salt).
- The structural formula is:
- Active Ingredient: bromocriptine mesylate, USP
- Inactive Ingredients: anhydrous lactose, colloidal silicon dioxide, magnesium stearate, maleic acid, povidone and pregelatinized starch.
Pharmacodynamics
- Bromocriptine mesylate produces its therapeutic effect in the treatment of Parkinson’s disease, a clinical condition characterized by a progressive deficiency in dopamine synthesis in the substantia nigra, by directly stimulating the dopamine receptors in the corpus striatum. In contrast, levodopa exerts its therapeutic effect only after conversion to dopamine by the neurons of the substantia nigra, which are known to be numerically diminished in this patient population.
Pharmacokinetics
- Absorption
- Following single dose administration of bromocriptine mesylate tablets, 2 x 2.5 mg to 5 healthy volunteers under fasted conditions, the mean peak plasma levels of bromocriptine, time to reach peak plasma concentrations and elimination half-life were 465 pg/mL ± 226, 2.5 hrs ± 2 and 4.85 hr, respectively. Linear relationship was found between single doses of bromocriptine mesylate and Cmax and AUC in the dose range of 1 to 7.5 mg. The pharmacokinetics of bromocriptine metabolites have not been reported.
- Food did not significantly affect the systemic exposure of bromocriptine following administration of bromocriptine mesylate tablets, 2.5 mg. It is recommended that bromocriptine mesylate be taken with food because of the high percentage of subjects who vomit upon receiving bromocriptine under fasting conditions.
- Following bromocriptine mesylate 5 mg administered twice daily for 14 days, the bromocriptine Cmax and AUC at steady state were 628 ± 375 pg/mL and 2377 ± 1186 pg*hr/mL, respectively.
- Distribution
- In vitro experiments showed that bromocriptine was 90%-96% bound to serum albumin.
- Metabolism
- Bromocriptine undergoes extensive first-pass biotransformation, reflected by complex metabolite profiles and by almost complete absence of parent drug in urine and feces.
- In vitro studies using human liver microsomes showed that bromocriptine has a high affinity for CYP3A and hydroxylations at the proline ring of the cyclopeptide moiety constituted a main metabolic pathway. Inhibitors and/or potent substrates for CYP3A4 might therefore inhibit the clearance of bromocriptine and lead to increased levels. The participation of other major CYP enzymes such as 2D6, 2C8, and 2C19 on the metabolism of bromocriptine has not been evaluated. Bromocriptine is also an inhibitor of CYP3A4 with a calculated IC50 value of 1.69 μM. Given the low therapeutic concentrations of bromocriptine in patients (Cmax=0.82 nM), a significant alteration of the metabolism of a second drug whose clearance is mediated by CYP3A4 should not be expected. The potential effect of bromocriptine and its metabolites to act as inducers of CYP enzymes has not been reported.
- Excretion
- About 82% and 5.6 % of the radioactive dose orally administered was recovered in feces and urine, respectively. Bromolysergic acid and bromoisolysergic acid accounted for half of the radioactivity in urine.5
- Special Populations
- Effect of Renal Impairment
- The effect of renal function on the pharmacokinetics of bromocriptine has not been evaluated.
- Since parent drug and metabolites are almost completely excreted via metabolism, and only 6% eliminated via the kidney, renal impairment may not have a significant impact on the PK of bromocriptine and its metabolites.
- Effect of Liver Impairment
- The effect of liver impairment on the PK of bromocriptine mesylate and its metabolites has not been evaluated. Since bromocriptine mesylate is mainly eliminated by metabolism, liver impairment may increase the plasma levels of bromocriptine, therefore, caution may be necessary.
- The effect of age, race, and gender on the pharmacokinetics of bromocriptine and its metabolites has not been evaluated.
Nonclinical Toxicology
- A 74-week study was conducted in mice using dietary levels of bromocriptine mesylate equivalent to oral doses of 10 and 50 mg/kg/day. A 100-week study in rats was conducted using dietary levels equivalent to oral doses of 1.7, 9.8, and 44 mg/kg/day. The highest doses tested in mice and rats were approximately 2.5 and 4.4 times, respectively, the maximum human dose administered in controlled clinical trials (100 mg/day) based on body surface area. Malignant uterine tumors, endometrial and myometrial were found in rats as follows: 0/50 control females, 2/50 females given 1.7 mg/kg daily, 7/49 females given 9.8 mg/kg daily, and 9/50 females given 44 mg/kg daily. The occurrence of these neoplasms is probably attributable to the high estrogen/progesterone ratio which occurs in rats as a result of the prolactin-inhibiting action of bromocriptine mesylate. The endocrine mechanisms believed to be involved in the rats are not present in humans. There is no known correlation between uterine malignancies occurring in bromocriptine-treated rats and human risk. In contrast to the findings in rats, the uteri from mice killed after 74 weeks of treatment did not exhibit evidence of drug-related changes.
- Bromocriptine mesylate was evaluated for mutagenic potential in the battery of tests that included Ames bacterial mutation assay, mutagenic activity in vitro on V79 Chinese hamster fibroblasts, cytogenetic analysis of Chinese hamster bone marrow cells following in vivo treatment, and an in vivo micronucleus test for mutagenic potential in mice.
- No mutagenic effects were obtained in any of these tests.
- Fertility and reproductive performance in female rats were not influenced adversely by treatment with bromocriptine beyond the predicted decrease in the weight of pups due to suppression of lactation. In males treated with 50 mg/kg of this drug, mating and fertility were within the normal range. Increased perinatal loss was produced in the subgroups of dams, sacrificed on day 21 postpartum (p.p.) after mating with males treated with the highest dose (50 mg/kg).
Clinical Studies
- In about 75% of cases of amenorrhea and galactorrhea, bromocriptine mesylate therapy suppresses the galactorrhea completely, or almost completely, and reinitiates normal ovulatory menstrual cycles.
- Menses are usually reinitiated prior to complete suppression of galactorrhea; the time for this on average is 6-8 weeks. However, some patients respond within a few days, and others may take up to 8 months.
- Galactorrhea may take longer to control depending on the degree of stimulation of the mammary tissue prior to therapy. At least a 75% reduction in secretion is usually observed after 8-12 weeks. Some patients may fail to respond even after 12 months of therapy.
- In many acromegalic patients, bromocriptine mesylate produces a prompt and sustained reduction in circulating levels of serum growth hormone.
How Supplied
- Bromocriptine Mesylate Tablets, USP are available containing 2.5 mg of bromocriptine (as the mesylate). The 2.5 mg tablets are white to off-white, round, bevel edged snap tablets, debossed with "PAD" over "0106" on one side and scored on the other side. Complies with USP dissolution test 1.
- Bottles of 30.........NDC 0574-0106-03
- Bottles of 100.......NDC 0574-0106-01
- Store at 20° to 25°C (68° to 77°F).
- Dispense in a tight, light-resistant container as defined in the USP using a child-resistant closure.
Storage
There is limited information regarding Bromocriptine Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Bromocriptine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Bromocriptine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- During clinical trials, dizziness, drowsiness, faintness, fainting, and syncope have been reported early in the course of bromocriptine mesylate therapy. In postmarketing reports, bromocriptine mesylate has been associated with somnolence, and episodes of sudden sleep onset, particularly in patients with Parkinson’s disease. Sudden onset of sleep during daily activities, in some cases without awareness or warning signs, has been reported very rarely. All patients receiving bromocriptine mesylate should be cautioned with regard to engaging in activities requiring rapid and precise responses, such as driving an automobile or operating machinery. Patients being treated with bromocriptine mesylate and presenting with somnolence and/or sudden sleep episodes must be advised not to drive or engage in activities where impaired alertness may put themselves or others at risk of serious injury or death (e.g., operating machines).
- Patients receiving bromocriptine mesylate for hyperprolactinemic states associated with macroadenoma or those who have had previous transsphenoidal surgery, should be told to report any persistent watery nasal discharge to their physician. Patients receiving bromocriptine mesylate for treatment of a macroadenoma should be told that discontinuation of drug may be associated with rapid regrowth of the tumor and recurrence of their original symptoms.
- Patients and their caregivers should be alerted to the possibility that they may experience intense urges to spend money uncontrollably, intense urges to gamble, increased sexual urges and other intense urges and the inability to control these urges while taking bromocriptine mesylate.
- Especially during the first days of treatment, hypotensive reactions may occasionally occur and result in reduced alertness, particular care should be exercised when driving a vehicle or operating machinery.
Precautions with Alcohol
- Alcohol-Bromocriptine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- BROMOCRIPTINE MESYLATE®[2]
Look-Alike Drug Names
There is limited information regarding Bromocriptine Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Jinno M, Yoshimura Y, Ubukata Y, Nakamura Y (1996). "A novel method of ovarian stimulation for in vitro fertilization: bromocriptine-rebound method". Fertil Steril. 66 (2): 271–4. PMID 8690115.
- ↑ "BROMOCRIPTINE MESYLATE- bromocriptine mesylate tablet".
{{#subobject:
|Page Name=Bromocriptine |Pill Name=Parlodel_NDC_00780017.jpg |Drug Name=Parlodel |Pill Ingred=BROMOCRIPTINE MESYLATE[BROMOCRIPTINE]|+sep=; |Pill Imprint=PARLODEL;2;12 |Pill Dosage=2.5 mg |Pill Color=White|+sep=; |Pill Shape=Round |Pill Size (mm)=8 |Pill Scoring=2 |Pill Image= |Drug Author=Novartis Pharmaceuticals Corporation |NDC=00780017
}}
{{#subobject:
|Page Name=Bromocriptine |Pill Name=Parlodel_NDC_00780102.jpg |Drug Name=Parlodel |Pill Ingred=BROMOCRIPTINE MESYLATE[BROMOCRIPTINE]|+sep=; |Pill Imprint=PARLODEL;5;mg;S |Pill Dosage=5 mg |Pill Color=Brown|+sep=; |Pill Shape=Capsule |Pill Size (mm)=16 |Pill Scoring=1 |Pill Image= |Drug Author=Novartis Pharmaceuticals Corporation |NDC=00780102
}}
{{#subobject:
|Page Name=Bromocriptine |Pill Name=Bromocriptine_Mesylate_NDC_03782042.jpg |Drug Name=Bromocriptine Mesylate |Pill Ingred=BROMOCRIPTINE MESYLATE[BROMOCRIPTINE]|+sep=; |Pill Imprint=M;42 |Pill Dosage=2.5 mg |Pill Color=White|+sep=; |Pill Shape=Round |Pill Size (mm)=7 |Pill Scoring=2 |Pill Image= |Drug Author=Mylan Pharmaceuticals Inc. |NDC=03782042
}}
{{#subobject:
|Page Name=Bromocriptine |Pill Name=Bromocriptine_Mesylate_NDC_03787096.jpg |Drug Name=Bromocriptine Mesylate |Pill Ingred=BROMOCRIPTINE MESYLATE[BROMOCRIPTINE]|+sep=; |Pill Imprint=MYLAN;7096 |Pill Dosage=5 mg |Pill Color=Brown;White|+sep=; |Pill Shape=Capsule |Pill Size (mm)=14 |Pill Scoring=1 |Pill Image= |Drug Author=Mylan Pharmaceuticals Inc. |NDC=03787096
}}
{{#subobject:
|Page Name=Bromocriptine |Pill Name=Bromocriptine_mesylate_NDC_05740106.jpg |Drug Name=Bromocriptine mesylate |Pill Ingred=Bromocriptine mesylate[Bromocriptine]|+sep=; |Pill Imprint=PAD;0106 |Pill Dosage=2.5 mg |Pill Color=White|+sep=; |Pill Shape=Round |Pill Size (mm)=8 |Pill Scoring=2 |Pill Image= |Drug Author=Paddock Laboratories, Inc. |NDC=05740106
}}
{{#subobject:
|Page Name=Bromocriptine |Pill Name=CYCLOSET_NDC_680120258.jpg |Drug Name=CYCLOSET |Pill Ingred=bromocriptine mesylate[bromocriptine]|+sep=; |Pill Imprint=C;9 |Pill Dosage=0.8 mg |Pill Color=White|+sep=; |Pill Shape=Round |Pill Size (mm)=6 |Pill Scoring=1 |Pill Image= |Drug Author=Santarus, Inc. |NDC=680120258
}}
{{#subobject:
|Label Page=Bromocriptine |Label Name=Bromocriptine02.png
}}
{{#subobject:
|Label Page=Bromocriptine |Label Name=Bromocriptine03.png
}}
{{#subobject:
|Label Page=Bromocriptine |Label Name=Bromocriptine04.png
}}