Organic peroxide
Overview
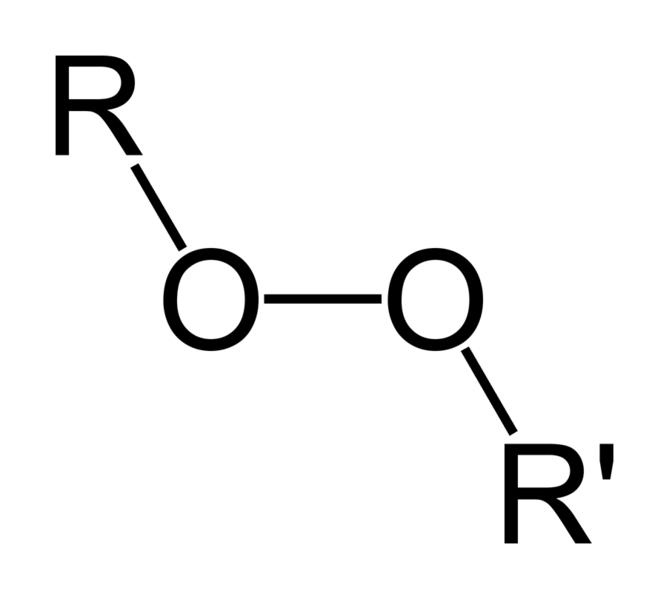
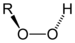
Organic peroxides are organic compounds containing the peroxide functional group (ROOR'). If the R' is hydrogen, the compound is called an organic hydroperoxide. Peresters have general structure RC(O)OOR.
The O-O bond easily breaks and forms free radicals of the form RO·. This makes organic peroxides useful as catalysts for some types of polymerisation, such as the epoxy resins used in glass-reinforced plastics. MEKP and benzoyl peroxide are commonly used for this purpose. However, the same property also means that organic peroxides can either intentionally or unintentionally initiate explosive polymerisation in materials with unsaturated chemical bonds, and this process has been used in explosives.[1]
Most organic peroxides are highly flammable, explosive materials, often powerful and volatile. As little as 5 milligrams of diethyl ether peroxide can shatter glass chemical apparatuses. Organic peroxides, like their inorganic counterparts, are powerful bleaching agents.
Occurrence and use
Organic peroxides find numerous uses in various industries, as accelerators, activators, catalysts, cross-linking agents, curing and vulcanization agents, hardeners, initiators and promoters.
Methyl ethyl ketone peroxide, benzoyl peroxide and to a much smaller degree acetone peroxide are used as radical initiators for radical polymerization of some resins, eg. polyester and silicone, often encountered when making fiberglass. Methyl ethyl ketone peroxide can oxidize acetone to acetone peroxide (an explosive), so mixing it with acetone is discouraged. Polymerization initiators are usually supplied as dilute solutions, but even commercial products, especially the more concentrated ones, may form crystals around the lid when older, making the can shock-sensitive.
Pinane hydroperoxide is used in production of styrene-butadiene synthetic rubber.
Benzoyl peroxide and acetone peroxide are used as bleaching and "maturing" agents for treating flour to make its grain release gluten easier; the alternative is letting the flour slowly oxidize by air, which is too slow for the industrialized era.
Benzoyl peroxide is a highly effective topical medication for treating most forms of acne.
Cumene hydroperoxide is an intermediate in the cumene process of industrial synthesis of phenol.
Acetone peroxide has became a favorite explosive of paramilitaries, because of its easy manufacture, despite its instability. It is notorious for its susceptibility to heat, friction, and shock.
Safety
Many organic chemicals form peroxides in presence of atmospheric oxygen and sometimes ultraviolet light; a typical example is the diethyl ether peroxide. As they combine unstably bound oxygen together with hydrogen and carbon in the same molecule, organic peroxides catch fire easily and burn rapidly and intensely. The same applies to organic materials contaminated with organic peroxides.
Since peroxides can form spontaneously in some materials, some caution must be exercised with such "peroxide forming materials". In addition, many liquid ethers in the presence of air, light, and metal slowly (over a period of months) form ether hydroperoxides and peroxides (e.g. diethyl ether peroxide) which are extremely unstable. Consequently it is recommended that ether be stored over potassium hydroxide, which not only destroys peroxides but also acts as a powerful desiccator (drying agent). Extreme care must be taken with samples showing signs of crystal growth or precipitates. The ease with which diethyl ether, tetrahydrofuran or ethylene glycol dimethyl ether form explosive peroxides is the reason why they tend to be avoided in industrial processes.
Caution must be executed with mixing peroxide-forming materials with oxidizing agents. Acetone peroxide, a powerful explosive, is an unwanted and dangerous byproduct of several chemical reactions, ranging from synthesis of MDMA (where it is a by-product of isosafrole oxidation in acetone) to industrial production of phenol (where the second product of the cumene process, acetone, is partially oxidized to peroxide on the second reaction step).
Accidental preparation of organic peroxides can occur by mixing ketone solvents (most commonly acetone) with waste materials containing hydrogen peroxide or other oxidizers and leaving the mixture standing for several hours.
Organic peroxides tend to react with metals. Glass, stainless steel, polyethylene or teflon containers are suggested for handling and storage; steel, copper alloys, rubber, lead, etc. should not be used. Empty containers must be washed with water immediately.
Organic peroxides must not be directly mixed with materials containing heavy metal ions like iron, cobalt or manganese, as these promote the decomposition of the peroxides. Direct mixing with amine compounds should be avoided as well. When mixing peroxides with amines (e.g., when making polyamides), both the peroxide and the amine have to be diluted separately with the monomer; the diluted chemicals can then be mixed.
Spillages of small amounts of organic peroxides can be wiped off with a rag, which then has to be disposed by burning at a safe place. Materials with absorbed organic peroxides must be saturated with water when stored.
Organic peroxides are sensitive to light and have to be stored in darkness. Some decompose at room temperature and release gaseous products; gas ejectors on the lids of the containers are required. Based on the Self Accelerating Decomposition Temperature, some peroxides have to be stored refrigerated.
In case of a fire, an explosion can be anticipated. A foam fire extinguisher can be used for small fires.
Small amounts of organic peroxides can be disposed of by careful burning of a substance diluted to under 10% or hydrolyzed. For hydrolysis, using a solution consisting of 80 parts water, 20 parts sodium hydroxide, and 0.3 parts surfactant to allow easier wetting of the crystals of the peroxide. The recommended amount of the solution is 10 times the weight of the peroxide. The peroxide has to be slowly poured into the solution, with constant stirring to avoid local overheating. The reaction is slightly exothermic, but additional cooling is not required. The reaction is slow, and requires stirring for some 12 to 24 hours; the peroxide can then be considered decomposed.
Synthesis
Peroxides can be synthesized in the laboratory in a number of ways:
- peroxy acids by oxidation of carboxylic acids with hydrogen peroxide
- peroxy acids by oxidation and hydrolysis of Grignard reagents
- peroxides by photooxidation of dienes
- peroxides by oxymercuration of alkenes followed by reaction with a hydroperoxide
- peroxy acids by reaction of alkyl halides with hydrogen peroxide
Reactions
Some peroxide reactions are:
- organic reduction to alcohols with lithium aluminium hydride or phosphite esters
- cleavage to ketones and alcohols in the base catalyzed Kornblum–DeLaMare rearrangement
See also
External links
References
- ↑ See for example the 2006 transatlantic aircraft plot.