Doxercalciferol (injection)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ammu Susheela, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Doxercalciferol (injection) is a vitamin analog that is FDA approved for the treatment of secondary hyperparathyroidism in patients with chronic kidney disease on dialysis. Common adverse reactions include weakness, headache, somnolence, nausea, vomiting, dry mouth, constipation, muscle pain, bone pain, metallic taste, anorexia, polyuria, polydipsia, weight loss, nocturia, conjunctivitis (calcific), pancreatitis, photophobia, rhinorrhea, pruritus, hyperthermia, decreased libido, elevated blood urea nitrogen (BUN), albuminuria, hypercholesterolemia, elevated serum aspartate transaminase (AST) and alanine transaminase (ALT), ectopic calcification, hypertension, cardiac arrhythmias, sensory disturbances, dehydration, apathy, arrested growth, urinary tract infection and, rarely, overt psychosis.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Doxercalciferol is indicated for the treatment of secondary hyperparathyroidismin patients with chronic kidney disease on dialysis.
- Adult Administration:
- For intravenous use only. The optimal dose of doxercalciferol must be carefully determined for each patient.
- The recommended initial dose of doxercalciferol is 4 mcg administered intravenously as a bolus dose three times weekly at the end of dialysis(approximately every other day). The initial dose should be adjusted, as needed, in order to lower blood iPTH into the range of 150 to 300 pg/mL. The dose may be increased at 8-week intervals by 1 to 2 mcg if iPTH is not lowered by 50% and fails to reach the target range. Dosages higher than 18 mcg weekly have not been studied. Drug administration should be suspended if iPTH falls below 100 pg/mL and restarted one week later at a dose that is at least 1 mcg lower than the last administered dose. During titration, iPTH, serum calcium, and serum phosphorus levels should be obtained weekly. If hypercalcemia, hyperphosphatemia, or a serum calcium times phosphorus product greater than 55 mg2/dL2 is noted, the dose of doxercalciferol should be decreased or suspended and/or the dose of phosphate binders should be appropriately adjusted. If suspended, the drug should be restarted at a dose that is 1 mcg lower.
- Dosing must be individualized and based on iPTH levels with monitoring of serum calcium and serum phosphorus levels. TABLE 5 presents a suggested approach in dose titration.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Doxercalciferol in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Doxercalciferol in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Doxercalciferol (injection) FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Doxercalciferol in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Doxercalciferol in pediatric patients.
Contraindications
- Doxercalciferol should not be given to patients with a tendency towards hypercalcemia or current evidence of vitamin D toxicity.
Warnings
- Overdosage of any form of vitamin D, including doxercalciferol is dangerous.
- Progressive hypercalcemia due to overdosage of vitamin D and its metabolites may be so severe as to require emergency attention. Acute hypercalcemia may exacerbate tendencies for cardiac arrhythmias and seizures and may potentiate the action of digitalis drugs.
- Chronic hypercalcemia can lead to generalized vascular calcification and other soft-tissue calcification. The serum calcium times serum phosphorus (Ca X P) product should be maintained at <55 mg2/dL2 in patients with chronic kidney disease.
- Radiographic evaluation of suspect anatomical regions may be useful in the early detection of this condition.
- Since doxercalciferol is a precursor for 1α,25-(OH)2D2, a potent metabolite of vitamin D2, pharmacologic doses of vitamin D and its derivatives should be withheld during doxercalciferol treatment to avoid possible additive effects and hypercalcemia.
- Oral calcium-based or other non-aluminum-containing phosphate binders and a low phosphate diet should be used to control serum phosphorus levels in patients undergoing dialysis. Uncontrolled serum phosphorus exacerbates secondary hyperparathyroidism and can lessen the effectiveness of doxercalciferol in reducing blood PTH levels. If hypercalcemia occurs after initiating doxercalciferol therapy, the dose of doxercalciferol and/or calcium-containing phosphate binders should be decreased. If hyperphosphatemia occurs after initiating doxercalciferol, the dose of doxercalciferol should be decreased and/or the dose of phosphate binders increased.
- Magnesium-containing antacids and doxercalciferol should not be used concomitantly in patients on chronic renal dialysis because such use may lead to the development of hypermagnesemia.
Adverse Reactions
Clinical Trials Experience
- Doxercalciferol Injection has been evaluated for safety in 70 patients with chronic renal disease on hemodialysis (who had been previously treated with oral doxercalciferol) from two 12-week, open-label, single-arm, multi-centered studies.
- Because there was no placebo group included in the studies of doxercalciferol Injection, TABLE 4 provides the adverse event incidence rates from placebo-controlled studies of oral doxercalciferol.
Postmarketing Experience
- Potential adverse effects of doxercalciferol are, in general, similar to those encountered with excessive vitamin D intake. The early and late signs and symptoms of vitamin D intoxication associated with hypercalcemia include:
Early
- Weakness, headache, somnolence, nausea, vomiting, dry mouth, constipation, muscle pain, bone pain, metallic taste, and anorexia.
Late
- Polyuria, polydipsia, anorexia, weight loss, nocturia, conjunctivitis (calcific), pancreatitis, photophobia, rhinorrhea, pruritus, hyperthermia, decreased libido, elevated blood urea nitrogen (BUN), albuminuria, hypercholesterolemia, elevated serum aspartate transaminase (AST) and alanine transaminase (ALT), ectopic calcification, hypertension, cardiac arrhythmias, sensory disturbances, dehydration, apathy, arrested growth, urinary tract infections, and, rarely, overt psychosis.
Drug Interactions
There is limited information regarding Doxercalciferol (injection) Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- Reproduction studies in rats and rabbits, at doses up to 20 mcg/kg/day and 0.1 mcg/kg/day (approximately 25 times and less than the maximum recommended human oral dose of 60 mcg/week based on mcg/m2 body surface area, respectively) have revealed no teratogenic or fetotoxic effects due to doxercalciferol.
- There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Doxercalciferol (injection) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Doxercalciferol (injection) during labor and delivery.
Nursing Mothers
- It is not known whether doxercalciferol is excreted in human milk. Because other vitamin D derivatives are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from doxercalciferol, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
There is no FDA guidance on the use of Doxercalciferol (injection) in pediatric settings.
Geriatic Use
- Of the 70 patients treated with doxercalciferol Injection in the two Phase 3 clinical studies, 12 patients were 65 years or over. In these studies, no overall differences in efficacy or safety were observed between patients 65 years or older and younger patients.
Gender
There is no FDA guidance on the use of Doxercalciferol (injection) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Doxercalciferol (injection) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Doxercalciferol (injection) in patients with renal impairment.
Hepatic Impairment
- Studies examining the influence of hepatic insufficiency on the metabolism of doxercalciferol were inconclusive. Since patients with hepatic insufficiency may not metabolize doxercalciferol appropriately, the drug should be used with caution in patients with impaired hepatic function. More frequent monitoring of iPTH, calcium, and phosphorus levels should be done in such individuals.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Doxercalciferol (injection) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Doxercalciferol (injection) in patients who are immunocompromised.
Administration and Monitoring
Administration
Monitoring
There is limited information regarding Doxercalciferol (injection) Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Doxercalciferol (injection) and IV administrations.
Overdosage
- Administration of doxercalciferol to patients in excess doses can cause hypercalcemia, hypercalciuria, hyperphosphatemia, and over-suppression of PTH secretion leading in certain cases to adynamic bone disease. High intake of calcium and phosphate concomitant with doxercalciferol may lead to similar abnormalities.
- High levels of calcium in the dialysate bath may contribute to hypercalcemia.
Treatment of Hypercalcemia and Overdosage
- General treatment of hypercalcemia (greater than 1 mg/dL above the upper limit of the normal range) consists of immediate suspension of doxercalciferol therapy, institution of a low calcium diet, and withdrawal of calcium supplements. Serum calcium levels should be determined at least weekly until normocalcemia ensues. Hypercalcemia usually resolves in 2 to 7 days. When serum calcium levels have returned to within normal limits, doxercalciferol therapy may be reinstituted at a dose that is at least 1 mcg lower than prior therapy. Serum calcium levels should be obtained weekly after all dosage changes and during subsequent dosage titration. Persistent or markedly elevated serum calcium levels may be corrected by dialysis against a reduced calcium or calcium-free dialysate.
Treatment of Accidental Overdosage of Doxercalciferol®
- The treatment of acute accidental overdosage of doxercalciferol should consist of general supportive measures. Serial serum electrolyte determinations (especially calcium), rate of urinary calcium excretion, and assessment of electrocardiographic abnormalities due to hypercalcemia should be obtained. Such monitoring is critical in patients receiving digitalis. Discontinuation of supplemental calcium and institution of a low calcium diet are also indicated in accidental overdosage. If persistent and markedly elevated serum calcium levels occur, treatment with standard medical care should be followed, as needed. Based on similarities between doxercalciferol and its active metabolite, 1α,25-(OH)2D2, it is expected that doxercalciferol is not removed from the blood by dialysis.
Pharmacology
There is limited information regarding Doxercalciferol (injection) Pharmacology in the drug label.
Mechanism of Action
- Calcitriol (1α,25-(OH)2D3) and 1α,25-(OH)2D2 regulate blood calcium at levels required for essential body functions. Specifically, the biologically active vitamin D metabolites control the intestinal absorption of dietary calcium, the tubular reabsorption of calcium by the kidney and, in conjunction with parathyroid hormone (PTH), the mobilization of calcium from the skeleton. They act directly on bone cells (osteoblasts) to stimulate skeletal growth, and on the parathyroid glands to suppress PTH synthesis and secretion. These functions are mediated by the interaction of these biologically active metabolites with specific receptor proteins in the various target tissues. In uremic patients, deficient production of biologically active vitamin D metabolites (due to lack of or insufficient 25-hydroxyvitamin D-1-alpha-hydroxylase activity) leads to secondary hyperparathyroidism, which contributes to the development of metabolic bone disease in patients with renal failure.
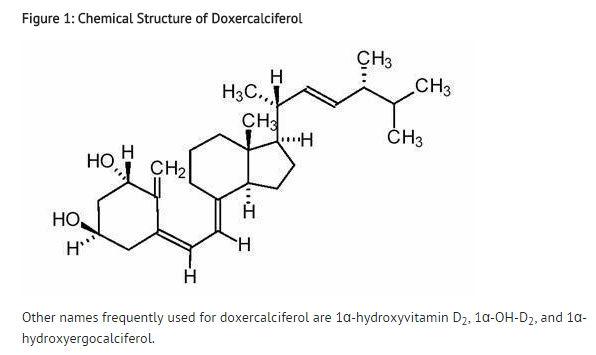
Structure
- Doxercalciferol, the active ingredient in doxercalciferol®, is a synthetic vitamin D2 analog that undergoes metabolic activation in vivo to form 1α,25-dihydroxyvitamin D2 (1α,25-(OH)2D2), a naturally occurring, biologically active form of vitamin D2. doxercalciferol is available as a sterile, clear, colorless aqueous solution for intravenous injection. Doxercalciferol single-use injection is supplied in a stoppered 2 mL amber glass vial containing either 4 mcg/2 mL or 2 mcg/mL. Each vial includes an aluminum seal and yellow (4 mcg/2 mL) or green (2 mcg/mL) flip-off cap. Each milliliter (mL) of solution contains doxercalciferol, 2 mcg; ethanol, 100%, 0.05 mL; Polysorbate 20, 10 mg; sodium chloride, 1.5 mg; butylated hydroxytoluene, 0.02 mg; sodium phosphate dibasic, heptahydrate, 14.4 mg; sodium phosphate monobasic, monohydrate, 1.8 mg; and disodium edetate, 1.1 mg. Doxercalciferol is also supplied as a multi-dose injection contained within a stoppered 2 mL amber glass vial containing 4 mcg/2 mL. Each vial includes an aluminum seal and an orange plastic flip-off cap. Each milliliter (mL) of solution contains doxercalciferol, 2 mcg; ethanol, 100%, 0.075 mL; Polysorbate 20, 10 mg; sodium chloride, 1.5 mg; butylated hydroxytoluene, 0.02 mg; sodium phosphate dibasic, heptahydrate, 14.4 mg; sodium phosphate monobasic, monohydrate, 1.8 mg; and disodium edetate, 1.1 mg.
- Doxercalciferol is a colorless crystalline compound with a calculated molecular weight of 412.66 and a molecular formula of C28H44O2. It is soluble in oils and organic solvents, but is relatively insoluble in water.
Pharmacodynamics
There is limited information regarding Doxercalciferol (injection) Pharmacodynamics in the drug label.
Pharmacokinetics
- After intravenous administration, doxercalciferol is activated by CYP 27 in the liver to form 1α,25-(OH)2D2 (major metabolite) and 1α,24-dihydroxyvitamin D2 (minor metabolite). Activation of doxercalciferol does not require the involvement of the kidneys.
- Peak blood levels of 1α,25-(OH)2D2 are reached at 8 +/- 5.9 hours (mean +/- SD) after a single intravenous dose of 5 mcg of doxercalciferol. The mean elimination half-life of 1α,25-(OH)2D2 after an oral dose is approximately 32 to 37 hours with a range of up to 96 hours. The mean elimination half-life in patients with end stage renal disease (ESRD) and in healthy volunteers appears to be similar following an oral dose. Hemodialysis causes a temporary increase in 1α,25-(OH)2D2 mean concentrations presumably due to volume contraction. 1α,25-(OH)2D2 is not removed from blood during hemodialysis.
Nonclinical Toxicology
There is limited information regarding Doxercalciferol (injection) Nonclinical Toxicology in the drug label.
Clinical Studies
- The safety and effectiveness of doxercalciferol Injection were evaluated in two open-label, single-arm, multi-centered clinical studies (Study C and Study D) in a total of 70 patients with chronic kidney disease on hemodialysis (Stage 5 CKD). Patients in Study C were an average age of 54 years (range: 23-73), were 50% male, and were 61% African-American, 25% Caucasian, and 14% Hispanic, and had been on hemodialysis for an average of 65 months. Patients in Study D were an average age of 51 years (range: 28-76), were 48% male, and 100% African-American and had been on hemodialysis for an average of 61 months. This group of 70 of the 138 patients who had been treated with doxercalciferol Capsules in prior clinical studies (Study A and Study B) received doxercalciferol Injection in an open-label fashion for 12 weeks following an 8-week washout (control) period. Dosing of doxercalciferol Injection was initiated at the rate of 4 mcg administered at the end of each dialysis session (3 times weekly) for a total of 12 mcg per week. The dosage of doxercalciferol was adjusted in an attempt to achieve iPTH levels within a targeted range of 150 to 300 pg/mL. The dosage was increased by 2 mcg per dialysis session after 8 weeks of treatment if the iPTH levels remained above 300 pg/mL and were greater than 50% of baseline levels. The maximum dosage was limited to 18 mcg per week. If at any time during the trial iPTH fell below 150 pg/mL, doxercalciferol Injection was immediately suspended and restarted at a lower dosage the following week.
Results
- Fifty-two of the 70 patients who were treated with doxercalciferol Injection achieved iPTH levels ≤ 300 pg/mL. Forty-one of these patients exhibited plasma iPTH levels ≤ 300 pg/mL on at least 3 occasions. Thirty-six patients had plasma iPTH levels < 150 pg/mL on at least one occasion during study participation.
- Mean weekly doses in Study C ranged from 8.9 mcg to 12.5 mcg. In Study D, the mean weekly doses ranged from 9.1 mcg to 11.6 mcg.
- Decreases in plasma iPTH from baseline values were calculated using as baseline the average of the last 3 values obtained during the 8-week washout period and are displayed in the table below. Plasma iPTH levels were measured weekly during the 12-week study.
How Supplied
Single-Use Vial
- Doxercalciferol injection) is supplied in single-use amber glass vials containing 4 mcg doxercalciferol in 2 mL of solution or 2 mcg in 1 mL of solution. The closure consists of a fluorocarbon-coated chlorobutyl stopper, with an aluminum seal and either a yellow (4 mcg/2 mL) or green (2 mcg/mL) plastic flip‑off cap. Discard unused portion of single-use vial.
- NDC 58468-0123-1 4 mcg/2 mL single-use vial
- NDC 58468-0126-1 2 mcg/mL single-use vial
Multi-Dose Vial
- Doxercalciferol is also supplied in multi-dose amber glass vials containing 4 mcg doxercalciferol in 2 mL of solution. The closure consists of a fluorocarbon-coated chlorobutyl stopper, with an aluminum seal and an orange plastic flip-off cap.
- NDC 58468-0127-1 4 mcg/2 mL multi-dose vial
Storage
- Single-Use Vial
- Store at 25°C (77°F): excursions permitted to 15-30°C (59-86°F)
- Protect from light.
Multi-Dose Vial
- Store unopened multi-dose vials at 25°C (77°F): excursions permitted to 15-30°C (59-86°F)
- Store opened multi-dose vials at 2-8°C (36-46°F)
- Protect from light.
Images
Drug Images
{{#ask: Page Name::Doxercalciferol (injection) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel





{{#ask: Label Page::Doxercalciferol (injection) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Doxercalciferol (injection) Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Doxercalciferol interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- HECTOROL ®[1]
Look-Alike Drug Names
There is limited information regarding Doxercalciferol (injection) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.