Chronic myelogenous leukemia medical therapy
|
Chronic myelogenous leukemia Microchapters |
|
Differentiating Chronic myelogenous leukemia from other Diseases |
|---|
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
Chronic myelogenous leukemia medical therapy On the Web |
|
American Roentgen Ray Society Images of Chronic myelogenous leukemia medical therapy |
|
Directions to Hospitals Treating Chronic myelogenous leukemia |
|
Risk calculators and risk factors for Chronic myelogenous leukemia medical therapy |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Shyam Patel [2] Associate Editor(s)-in-Chief: Badria Munir M.B.B.S.[3]Mohamad Alkateb, MBBCh [4] "sandbox:SN"
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [5]; Associate Editor(s)-in-Chief:
Overview
Pernicious anemia (also called Addison's anemia) is a type of red blood cell disorder caused by impaired vitamin B12 metabolism. Vitamin B12 is primarily absorbed by the small intestine, after being bound to intrinsic factor secreted by parietal cells of gastric mucosa. When this process is disrupted by conditions like atrophic gastritis, celiac disease, small bowel resection etc, B12 deficiency ensues.
Historical perspective
- Pernicious anemia was first discovered by Thomas Addison, hence it is also known as addison's anemia.
- Loss of life from large volume blood loss in the people fighting in the first world war inspired George Whipple to investigate blood forming components such as arsenic, iron pills etc, but found liver to be the most effective. He bled dogs until they had clinical anemia and fed them cooked liver which showed an improvement in symptoms and hematopoeisis. [1]
- In 1948, Smith, Rickles et al., isolated the anti-pernicious factor from liver extract and named it Vitamin B12. They showed that even small amounts of this factor can be used to treat and to prevent pernicious anemia. [2]
Pathophysiology
Vitamin B12 is an essential vitamin for humans and animals because we cannot synthesise it on our own. B12 is a cofactor in DNA synthesis and other important biochemical reactions. Vitamin B12 deficiency manifests as anemia because hematopoetic stem cells in the bone marrow which are rapidly dividing need B12 for division and DNA production. This process is impaired leading to ineffective hematopoeisis. Vitamin B12 is also necessary for production of myelin which is an important component in the covering sheath of nerves. Deficiency results in improper nerve conduction due to nerve destabilisation. [3]
Physiology
- Vitamin B12 is also called cobalamin because it contains cobalt at the core of its structure. Dietary sources of vitamin B12 include meat, fish and eggs.[4]
- When consumed through its dietary source, B12 is bound to protein till it enters the stomach.
- In the stomach, B12 is uncoupled from its carrier protein due to the presence of gastric acid, which is why vitamin B12 deficiency is so commonly seen among those on chronic antacid medication. [5]
- Once in the stomach, it is then bound to gastric R binder, a glycoprotein secreted by the salivary glands till it reaches the duodenum.[6]
- In the duodenum and jejunum, the pancreatic enzymes digest the gastric R binder and cobalamin is bound to intrinsic factor (IF).
- Intrinsic factor is secreted by the gastric parietal cells. Once bound to IF, vitamin B12 travels up to the ileum where IF is removed and B12 binds with carrier proteins called transcobalamins and this complex is taken up by the liver and bone marrow, among other tissues.
- Inside the cells, the transcobalamin-B12 complex is dissolved and cobalamin is reduced to methylcobalamin which serves as a cofactor and coenzyme in many important biochemical reactions[7].
The two major reactions involving B12 in the human body are:
- Vitamin B12 in the from of cyanocobalamin is required in the synthesis of methionine. Methionine is produced from homocysteine and is catalysed by the enzyme methionine synthase. This enzyme utilises cyanocobalamin as a cofactor. Deficiency of vitamin B12 causes a decreased production of methionine and buildup of homocysteine. Hyperhomocysteinemia is implicated as a risk factor in cardiovascular disease.[8]
- The Kreb's cycle utilises vitamin B12 in the reaction converting methylmalonyl-CoA to succinyl-CoA. Thus vitamin B12 deficiency causes a buildup of methylmalonic acid, the substrate for the enzyme methylmalonyl coenzyme A mutase. Methylmalonic acid levels are elevated in the urine of people affected with pernicious anemia and other forms of B12 deficiency.
Storage
The human body can store anywhere from 2-5mg of vitamin B12. Most of this is stored in the liver and is recycled via enterohepatic circulation.
Pathogenesis
Pernicious anemia is a type of megaloblastic anemia caused due to improper vitamin B12 absorption by the body. Impaired absorption occurs because of deficiency of intrinsic factor which is produced by the parietal cells of the stomach. The etiology of pernicious anemia can be due to autoimmune causes or genetic disease. In autoimmune disease, the antibodies attack most of the gastric mucosa, but the antrum is spared.
Autoimmune causes of pernicious anemia
This is the most common cause of pernicious anemia. In autoimmune pernicious anemia, the body produces antibodies against parietal cells or intrinsic factor.
- Antibodies against parietal cells of the gastric mucosa work to inhibit the H+/K(+)-ATPase which is the proton pump present in the parietal cells. The proton pump serves as an auto antigen and activates the cytotoxic CD4+ T cells which proceed to destroy gastric mucosal cells.[9][10]
- Intrinsic factor antibodies are present in fewer cases of pernicious anaemia but are highly specific. There are 2 types of IF antibodies. They prevent the binding and absorption of cobalamin in the ileum via its receptor.[11]
Clinical features
- The symptoms of pernicious anemia take months, and often years to manifest. Patients most commonly present with symptoms of anemia like lightheadedness, dizziness, shortness of breath etc. The population affected with pernicious anemia is usually the elderly (>60 years) owing to its insidious onset.
- Pernicious anemia has hematological, gastrointestinal and neurological manifestations.
- Hematological signs are the earliest manifestation of the disease while neurological signs are seen much later.
- Patients with pernicious anemia usually have very low levels of hydrochloric acid in the stomach (achlorhydria) and high levels on gastrin (hypergastrinemia).
Differentiating pernicious anemia from other diseases
Pernicious anemia shares many similarities with other forms of megaloblastic anemia like B12 and folate deficiency.
- Vitamin B12 deficiency due to insufficient intake (eg veganism) has all the features of pernicious anemia like megaloblasts, hypersegmented neutrophils, neuropsychiatric manifestations. But atrophic gastritis is absent, so achlorhydria, parietal cell antibodies or IF antibodies are absent. Intrinsic factor levels are also normal.[6]
- Folic acid deficiency also results in megaloblastic anemia and similar hematological changes as pernicious anemia, but urinary excretion of methylmalonic acid is absent, so are features of pernicious anemia like achlorhydria, antibodies and normal IF levels.
- Ileal resection causes B12 deficiency due to decreased absorption.
- Certain drugs such as methotrexate, azathioprine cause folate deficiency and result in megaloblastic anemia. This is usually seen in patients taking chemotherapy or other chronic conditions such as rheumatoid arthritis. [12]
- Chronic proton pump inhibitor therapy also results in B12 deficiency as vitamin B12 cannot dissociate from its carrier protein in the absence of an acidic environment.[13]
- Long term use of metformin, such as in diabetics, is linked to vitamin B12 deficiency and symptoms similar to pernicious anemia, but this can be differentiated from pernicious anemia as it is seen in diabetics on chronic therapy.[14]
Associated Conditions
People affected with pernicious anemia might have other coexisting autoimmune conditions such as autoimmune thyroiditis, autoimmune diabetes, vitiligo etc. Autoimmune thyroiditis is most commonly seen in patients with pernicious anemia, particularly females. HLA DR3 has been implicated in the development of autoimmune diseases such as pernicious anemia[15].
Epidemiology and demographics
- Pernicious anemia is a disease of the elderly. The mean age of patients who are symptomatic is >60.[16]
- An exception is the genetic form of the disease which is a congenital deficiency of intrinsic factor and is seen in children <10 years of age.
- Men and women are equally affected
- Prevalence of pernicious anemia is estimated at 0.1% of the population.[17]
Genetics
- Some forms of pernicious anemia are congenital and a genetic link has been postulated because of a higher incidence in certain populations.
- Affected people have a complete or near total absence of intrinsic factor and the presence of antibodies against intrinsic factor.
- The genetic variant is transmitted through an autosomal recessive pattern.[18]
Risk factors
- People who have autoimmune conditions like diabetes mellitus, autoimmune thyroiditis are at higher risk of developing pernicious anemia.
Natural History, Complications and Prognosis
- In most cases, patients affected with pernicious anemia remain asymptomatic for many years.
- Early manifestations include fatigue, shortness of breath, pallor and weakness.
- Long standing untreated pernicious anemia results in irreversible neurological damage such as subacute combined degeneration of the spinal cord.
- Neurological changes are irreversible once they set in and do not resolve with cobalamin supplementation.
Diagnosis
A diagnosis of pernicious anemia is made by a history and physical examination, along with hematological and neurological examination.
Diagnostic criteria
- The only specific criteria to diagnose pernicious anemia is an intrinsic factor output of less than 200U/h after pentagastrin stimulation, where normal levels would be >2000U/h. [19]
Symptoms
Symptoms of pernicious anemia are summarised below
| Hematological symptoms | Gastrointestinal symptoms | Neurological symptoms |
|---|---|---|
| Fatigue | Loss of appetite | Parasthesias |
| Weakness | Weight loss
|
Depression |
| Shortness of breath | Nausea | Gait problems |
| Dizziness | Burning sensation on tongue | Weakness |
| Tachycardia | Diarrhea | Loss of balance |
| Lightheadedness | Vomiting | Confusion |
Physical examination findings
Most important physical examination findings are the neurological findings of long standing B12 deficiency which leads to subacute combined degeneration of the spinal cord.
- Hematological signs include pallor and icterus.[20]
- Neurological signs: Vitamin B12 deficiency causes nerve demyelination. B12 deficiency also causes a buildup of methylmalonic acid which is toxic to neuronal cells and causes apoptosis.[21].
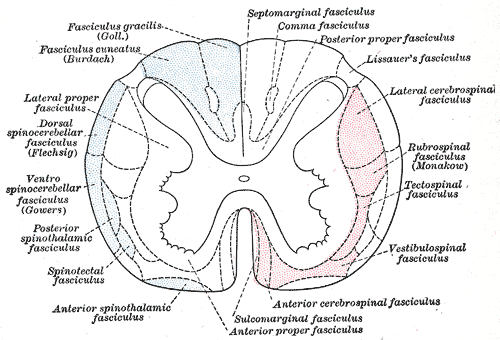
The main neurological manifestation of pernicious anemia and vitamin B12 deficiency is subacute combined degeneration. The posterior and lateral columns of the spinal cord are affected. Lateral column demyelination manifests as hyperreflexia and spasticity, while posterior column defects are loss of proprioception and vibration sense. Ataxia and loss of tandem gait are also manifestations of posterior column demyelination. Recreational or accidental inhalation of nitrous oxide gas (laughing gas) can precipitate subacute combined degeneration in people with low levels of vitamin B12.[22]
- Gastrointestinal signs: Upto 25% of people affected with pernicious anemia develop glossitis. The tongue appears red, "beefy" and smooth due to atrophy and blunting of the lingual papillae.[23]
Subacute combined degeneration
Laboratory findings
- The first step in diagnosis is a blood vitamin B12 level. Blood levels less than 200 pg/ml are seen in pernicious anemia.
- Intrinsic factor antibodies and Parietal cell antibodies.
- Low intrinsic factor level.[24]
- Gastric mucosal sampling shows parietal cell atrophy with antral sparing.[25]
- Increased level of gastrin.
- Increased levels of homocysteine and methylmalonyl-CoA.
- Decreased folate levels are seen due to "folate trapping" in the form of methyltetrahydrofolate.
Shilling Test
The Shilling test is no longer done to detect an IF deficiency but has historical importance. After a vitamin B12 deficiency is noted, the patient is given radioactively tagged cobalamin to take orally. Soon after this step, the patient is injected with unlabelled cobalamin intramuscularly. Urine is checked for radioactive cobalamin for the next 24 hours. In pernicious anemia, there is an intrinsic factor deficiency, therefore the orally consumed radioactive cobalamin will not be absorbed and can be detected in the urine. In the next step, the patient is given radioactive cobalamin along with intrinsic factor and their urine is checked for traces of radioactive cobalamin. Absence of radioactive cobalamin in the urine points to the deficiency of intrinsic factor in the patients stomach which is the cause of vitamin B12 deficiency[26]. If the cobalamin absorption does not increase even with intrinsic factor supplementation, patient can be given a course of antibiotics as bacterial overgrowth may hinder absorption.
Peripheral smear findings
- The most obvious peripheral smear finding is megaloblasts and macrocytes.
Megaloblastic anemia results due to the lagging behind of nuclear development when compared to cytoplasmic development. This is known as nuclear-cytoplasmic asynchrony. Such defective cells are destroyed in the bone marrow (intramedullary hemolysis).
- Decreased number of RBCs (erythopenia)
- Macrocytosis- the RBCs in pernicious anemia are very large. Macrocytosis is defined as cells that have an MCV >100 femtolitres (normal :80-100fL)
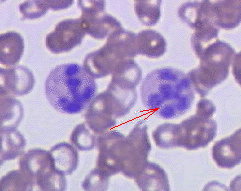
- Hypersegmented neutrophils : Neutrophils containing ≥ 6 lobes. [27]
- Poikilocytosis and anisocytosis
- Low reticulocyte count (reticulopenia)
- Howell-Jolly bodies
-
Atrophic gastritis
-
Hypersegmented neutrophil
Treatment
- Standard treatment for pernicious anemia is replacement of cobalamin via intramuscular injection. [28]
- 1000 mcg IM everyday for one week, followed by weekly injections the next month and then monthly once injections.
- Response to treatment is measured by an increase in reticulocyte count within 5 days of starting therapy.
- Patient also experience a sense of wellbeing shortly after beginning therapy.
- If reticulocytosis is not observed within the first week of therapy, other factors such as hypothyroidism, folate deficiency should be considered.
- Intramuscular therapy can be replaced by high dose oral therapy.[17]
- Neurological disease always warrants parenteral treatment.
- Within the first 3-4 weeks of treatment, marrow changes revert and there is resolution in macrocytosis.
- Most patients require lifelong monthly therapy.
- Routine follow up should be done with a CBC every few months.
- A small percentage of patients develop gastric carcinoma, particularly in the elderly. Regular surveillance helps in early detection and treatment. [29]
Prevention
- There is no primary preventive measure for pernicious anemia.
- Once sucessfully diagnosed and treated, patients with pernicious anemia are followed up every year for development of stomach cancer[30], or symptoms of anemia.
References
Overview
Medical therapies for chronic myelogenous leukemia (CML) include tyrosine kinase inhibitors, chemotherapy, stem cell transplant, and/or biological therapy. Improved understanding of the nature of the BCR-ABL protein and its action as a tyrosine kinase, targeted therapies have been developed (the first of which was imatinib mesylate) which specifically inhibit the activity of theBCR-ABL protein. These tyrosine kinase inhibitors can induce complete remissions in chronic myelogenous leukemia, confirming the central importance of BCR-ABL as the cause of chronic myelogenous leukemia. In patients with blast phase CML, cytotoxic chemotherapy is usually indicated.
Medical Therapy
- Medical therapy depends on the phase of chronic myelogenous leukemia.
- It s primarily treated with tyrosine kinase inhibitors (TKI).
- However, even with TKI therapy, CML can progress from chronic stable phase to a more aggressive pattern (accelerated phase), during which time disease control is tiresome to achieve.
- Consequently, it can result in an acute leukemia, called as blast crisis," which is generally refractory to treatment.[31][32][33][34][35]
Chronic Phase
Targeted therapy: Targeted therapy is the primary treatment for most people with chronic myelogenous leukemia in the chronic phase:
These are tyrosine kinase inhibitors (TKIs) that can be used for initial treatment of CML.
Imatinib:
- It is the primarily the standard first-line therapy for CML.
- Imatinib is efficacious in chronic phase (CP) and is less expensive than other tyrosine kinase inhibitors (TKIs).
- Compared with imatinib, second generation TKIs achieve faster responses, but all TKIs tend to accomplish effective long-term outcomes.
- Imatinib is more effective than non-TKI medications (eg, interferon [IFN] alfa plus cytarabine) in this setting.
Dose of imatinib:
- For patients with chronic phase, imatinib is started at 400 mg daily by mouth and is continued indefinitely for those who tolerate it and continue to respond by molecular and/or cytogenetic measures.[36]
- More than 60 percent of patients can remain on imatinib for at least five years.
Side effects of imatinib:
- Side effects are generally mild or moderate.
- The most common adverse effects are:[37]
- Edema (60%)
- Nausea (55%)
- Muscle cramps (50%)
- Rash (30%)
- Diarrhea (30%)
- Grade 3/4 neutropenia
- Leukopenia
- Thrombocytopenia
- Anemia
Impact of imatinib on survival rate:
- With a present follow-up of 5 years, the survival rate with imatinib is 87%. [38]
- The estimated 5-year survival rate is 89%, and survival without transformation rate is 93%.
- The impact of imatinib on survival rate in different phases of disease is presented in the following table.[39][40][41]
| Disease phase | Imatinib daily dose (mg) | Estimated survival rates at (X) years |
|---|---|---|
| Chronic Phase, newly diagnosed | 400 | 89% (5) |
| Chronic phase, previously diagnosed | 400 | 86% (4) 79% (5) |
| Accelerated phase | 400 vs 600 | 44% (3) vs 66% (3)
53% (4) |
| Blastic phase | 400-600 | 17% (2); 14-17% (2-3) |
Resistance to imatinib:
- Resistance to imatinib is defined as a failure to achieve complete hematologoic response at 3 months, a cytogenetic response at 6 months, or a major cytogenetic response at 12 months.[42][43]
- Recent recommendations of definitions of resistance versus suboptimal response to imatinib have been proposed. [42]
Definition of sub optimal response
- Lack of major molecular response may be considered as suboptimal response rather than failure as stated below in table :[41]
| Time on imatinib | Response | |
|---|---|---|
| Failure | Suboptimal | |
| 3 Months | No HR | No CHR |
| 6 Months | No CHR
Ph+100% |
Ph+ > 35% |
| 12 Months | Ph+>35% | Ph+> 5% |
| 18 months | Ph+>5% | No MMR < 3 months reduction of BCR-ABL/ABL |
| Any months | Loss of CHR
Loss of cytogenetic response Mutation |
Clonal Evaluation
Loss of MMR Mutation |
HR: Hematologic response; CHR: Complete Hematologic response; MMR: Major molecular response.
- Resistance to imatinib is often attributed to the emergence of clones expressing mutant forms of BCR-ABL, in which amino acid substitutions in the ABL kinase domain impair imatinib binding but retain kinase activity.[44][45][46][47]
- Initial reports suggested these mutations to be present in up to 90% of patients failing imatinib therapy; recent studies suggested rates of 40%.[48]
- Other proposed mechanisms of imatinib resistance include:[49][50]
- Amplification of the fusion gene
- Overexpression of the BCR-ABL oncoprotein
- Overexpression of molecules downstream of BCR-ABL signaling, such as Lyn kinase
- Clonal evolution, expression of the multidrug resistance phenotype
- Binding of a1 acid glycoprotein
- In addition, leukemic stem cell quiescent and insensitive to imatinib may cause persistence of CML.
- The estimated 4-year resistance rates are 20% in later chronic phaseand 70% to 90% in accelerated-blastic phases.
Dasatinib
- Dasatinib is an oral, second generation TKI.
- It is 350 times more potent than imatinib in vitro.[51][36] [52]
- It also inhibits the Src family of kinases, which may be important in blunting critical cell signaling pathways.[53]
- Though initially evaluated in patients in the salvage setting, it was later compared to imatinib in frontline CML to test the possibility that frontline use of the more potent TKIs might improve outcomes.
- A five-year follow-up showed that dasatinib induced more rapid and deeper responses at early time points compared to imatinib.[54]
- At 3 months, a higher proportion of patients treated with dasatinib achieved BCR-ABL1 transcripts
- Transformations to CML-BP were fewer in patients treated with dasatinib versus imatinib (4.6% versus 7.3%).
- However, the 5-year survival was similar with dasatinib and imatinib (91%, and 90%)
- There is more toxicity experienced in the dasatinib arm (Grades 3 or 4 adverse events 58% with dasatinib and 35% imatinib), mostly hematologic toxicity.[55]
- Pleural effusions occurred more frequently on dasatinib (19% versus <1%).
- Other side effects of dasatinib included myelosuppression (20%), and rare pulmonary hypertension(1–2%).
| Disease | No. | Response | |||
|---|---|---|---|---|---|
| CHR | Hematologic | Cytogenic response | |||
| Chronic | 387 | 90 | 00 | 51 | 40 |
| Accelerated | 175 | 34 | 59 | 34 | 25 |
| Blastic Lymphoid | 109 | 25 | 49 | 31 | 25 |
| Blastic Myeloid | 48 | 29 | 33 | 44 | 38 |
| Ph+ALL | 46 | 33 | 39 | 46 | 48 |
Dose of dasatinib:
- 100 mg daily was found as effective as 140 mg daily, with a better safety profile.[56]
Side Effects of dasatinib:
- There was no substantial difference in grade 3/4 toxicities by treatment schedule.
- Thirty-five percent of patients required dose reductions, although the median dose for all patients at 12 months was 100 mg/day (range: 20 to 100).
- Grade 3/4 toxicities included:
- Neutropenia (21%)
- Thrombocytopenia (10%)
- Pleural effusions occurred in eight patients (13%)
- In addition, the safety profile was favorable; only one patient discontinued therapy (subdural hematoma unlikely related) and one patient had his dose reduced to 20 mg daily due to pleural effusion.
- Such strategy will have a significant impact on our future practice.
Nilotinib:
- Nilotinib is a structural analog of imatinib.
- Its affinity for the ATP binding site on BCR-ABL1 is 30–50 times more in vitro.[57]
- Like dasatinib, nilotinib initially demonstrated the ability to induce hematologic and cytogenetic responses in patients who had failed imatinib.[58]
- The primary end point was the rate of major molecular response at 12 months.
- This endpoint was achieved at statistically significantly higher rates for both doses of nilotinib compared with imatinib (44% and 43% versus 22%, P<.001).
| No. Treated | Chronic | Accelerated | Blastic | Ph+ALL |
|---|---|---|---|---|
| % CHR/HR | 69/— | 16/40 | 4/13 | 27/33 |
| % Cytogenetic response | 68 | 56 | 29 | - |
| Complete | 34 | 16 | 21 | - |
| Partial | 14 | 12 | 8 | - |
Bosutinib:
- Bosutinib is an inhibitor of ABL and SRC kinases that is approved for initial treatment of CML in CP.[59]
- When compared with imatinib in newly diagnosed CML, bosutinib produces faster and slightly superior response rates.
- It is associated with more diarrhea and abnormal liver function tests.
- When compared with imatinib, bosutinib resulted in faster cytogenetic responses but similar rates of CCyR at 12 and 24 months, and a higher rate of major molecular response at 12 months, but not at 24 months.[60]
Side Effects of bosutinib:
- Bosutinib was associated with following:
- Diarrhea
- Vomiting
- Abdominal pain
- Edema
- Bone pain
- Muscle spasms
- Elevated serum transaminases
- Any grade toxicity can occur
- Bosutinib was also associated with a higher rate of drug discontinuation due to adverse events
- The overall toxicity profile was improved compared to the BELA trial , which utilized a dose of 500 mg of bosutinib, and led to US Food and Drug Administration approval of 400 mg bosutinib as initial therapy for CP CML.
- Compared with imatinib, bosutinib resulted in higher rates of MMRti and CCyR at 12 months, and achieved these milestones more quickly.[60]
Ponatinib:
- Ponatinib is a third-generation TKI that is specifically effective in patients who have the T3 resistance mutation in BCR-ABL. The T315I mutation is characterized by the conversion of threonine to isoleucine at the 315th position. It is considered as a tyrosine kinase domain (TKD) mutation. Ponatinib is the only effective therapy for this mutation.[61]
Selection of initial TKI
- The selection of a TKI for initial treatment of CML in chronic is determined by the following:
- Prognostic score
- Side effect profiles
- Comorbid illnesses
- Cost
- The following suggestions for treatment of chronic phase CML are influenced by the CML risk score (eg, EUTOS, Euro [Hasford], or Sokal scores):
Low- or intermediate-risk CML
- For most patients with low- or intermediate-risk chronic phase CML, it is advised that the treatment with any of the first or second generation TKIs (eg, imatinib, dasatinib, nilotinib, bosutinib).
- In this setting, side effect profiles, comorbid illnesses, and cost are important factors to the choice of TKI.
- Imatinib, which is available as a generic drug, is a reasonable choice for most patients and is preferred when cost is an important factor.
- Second generation TKIs may be preferred by clinicians and/or patients who place a higher value on attaining a quicker response.
- For patients with high-risk chronic phase CML, it is best to use second generation TKIs because, compared with imatinib, early molecular responses occur more frequent with second generation TKIs, and EMR is associated with improved survival in this setting.
- Choice of a second generation TKI is based on side effect profile and/or comorbid conditions. As examples, dasatinib might be preferred in a patient with a history of following:
- On the contrary, nilotinib is selected for a patient with a history of pleural or pericardial disease or effusions and avoided in patients with a history of cardiovascular diseases.
- Long-term follow-up of patients initially treated with imatinib suggests that more than half will remain on imatinib at five years; while some patients taking imatinib have persistent low-grade side effects (fatigue, arthralgias, and diarrhea), new toxicities have not emerged with longer follow-up.[62]
- In contrast, despite shorter follow-up, second generation TKIs are associated more severe, late toxicities (eg, pulmonary arterial hypertension with dasatinib; higher rates of thrombotic events [myocardial infarction, stroke, peripheral arterial occlusive disease] with nilotinib).[63]
- Patients on imatinib have excellent disease control and survival rates if clinicians monitor disease response and switch to a second generation TKI upon failure to meet a targeted response or with the development of drug intolerance.[64]
- The prices of TKIs differ substantially and vary by country.[65]
- In addition, the out-of-pocket expense for an individual depends upon their health care plan. Adherence is an independent predictor of response and is lower among patients with higher medication co-payments.[66]
Hematopoietic stem cell transplantation
- Stem cell transplant may be offered for CML in the chronic phase.
- It is sometimes used as a primary treatment option for younger people who have an HLA-matched donor.
- It is a treatment option for some people who do not achieve a complete response or develop resistance to or relapse with imatinib.
- Allogeneic hematopoietic cell transplantation (HCT) is a curative treatment option that comes at the cost of increased toxicity.
- A reduced intensity transplant may be an option for older people who may not tolerate a standard transplant.
- The chemotherapy or radiation used to prepare for a stem cell transplant is less intense than that used for a standard allogeneic transplant.
- Outcomes with HCT and tyrosine kinase inhibitors have not been directly compared in a well-designed trial.
- However, based on historical comparisons, HCT is associated with increased morbidity and mortality, despite the possibility of cure.
- Thus, allogeneic HCT is currently not offered as initial therapy, except in rare circumstances.
- With HCT, the probability of survival can be predicted with reasonable accuracy using a scoring system devised by the European Group for Blood and Marrow Transplantation .[67]
- The five-year overall survival rates for patients in the best risk groups ranged from 60 to 80 percent.
- As an example, a prospective trial of HCT in patients in first chronic phase, most of whom had failed to respond to initial treatment with imatinib, reported a transplant-related mortality rate of 8 percent.[68]
Aims of therapy and monitoring the response:
- The aim of initial therapy with a tyrosine kinase inhibitor is to achieve the milestones, which are optimal responses proposed by the European LeukemiaNet.[69]
- Failure to achieve these milestones should be confirmed with repeat studies before changes in therapy are initiated.
- A decision to change therapy must also take into consideration the trends in these values over time.
- Discontinuation of a TKI would be an option for only the approximately 15 to 20 percent of patients who achieve sustained non-detectable levels of BCR/ABL1 for at least two years; successful long-term discontinuation of therapy would be expected in less than 10 to 15 percent of newly diagnosed patients.[70]
- For individuals who have had a deep molecular remission, about half will have evidence of molecular recurrence and progression within one year after discontinuing TKI therapy, since viable CML stem cells can remain in a quiescent state in the marrow.[71][72].
- In general, the patients who are most likely to maintain a long treatment-free remission are those who have had the longest period of TKI treatment and have had undetectable transcript levels for years.
Biological therapy:
- Biological therapy may be offered for chronic stage CML. Biological therapy can be used alone or in combination with chemotherapy.
- The most common biological therapy used is interferon alfa (Intron A, Roferon A).
- Interferon alfa may be used for people who cannot tolerate, or whose CML is resistant to, imatinib.
- Chemotherapy may be offered for CML in the chronic phase.
- The types of chemotherapy used are:
- Hydroxyurea (Hydrea, Apo-hydroxyurea, Gen-hydroxyurea)
- Cytarabine (Cytosar)
- May be used in combination with interferon alfa
- Busulfan (Myleran [oral], Busulfex [intravenous])
Accelerated Phase
- Allogeneic transplant is preferred, but autologous transplant can also be done.
- Clinicians usually prefer that the leukemia returns to the chronic phase or is controlled before the transplant.
- A reduced-intensity transplant may be an option for older people who may not tolerate a standard transplant.
- The chemotherapy or radiation used to prepare for a stem cell transplant is less intense than that used for a standard allogeneic transplant.
- Targeted therapy
- Targeted therapy with a tyrosine kinase inhibitor may be offered during the accelerated phase of CML. For those already taking targeted therapy, the dose may be increased. The types of targeted therapy used are:
- Biological therapy
- Interferon alfa
- Cytarabine
- Chemotherapy may be offered for CML in the accelerated phase.
- The types of chemotherapy used are:
- Cytarabine
- HDAC (high-dose cytarabine)
- Hydroxyurea
- Busulfan
- Busulfex
Blast Phase
- Targeted therapy[35]
- Targeted therapy with a tyrosine kinase inhibitor may be offered for CML in the blast phase. For those already taking targeted therapy, the dose may be increased. The types of targeted therapy used are:
- The most common drugs used when the leukemia cells appear like AML (myeloid blast crisis) include:
- Cytarabine
- HDAC (high-dose cytarabine)
- An anthracycline, such as daunorubicin or doxorubicin
- Tُhioguanine
- Hydroxyurea
- The most common drugs used when the leukemia cells look like ALL (lymphoid blast crisis) include the drugs listed above as well as:
- There is increased risk of spread to the central nervous system (CNS) during the blast phase, so the following chemotherapy drugs may be given into the spinal fluid (intrathecal):
- Allogeneic stem cell transplant
- Radiation therapy may be offered for blast phase CML for:
- Splenomegaly
- Bone pain
Relapsed or Refractory Chronic Myelogenous
- Targeted therapy with a tyrosine kinase inhibitor may be offered for relapsed or refractory CML. For those already taking targeted therapy, the dose may be increased. The types of targeted therapy used are:
Supportive Therapy
- Antibiotics and Antifungals
- Blood products
- Granulocyte colony-stimulating factors (G-CSF)
References
- ↑ Sinclair L (2008). "Recognizing, treating and understanding pernicious anaemia". J R Soc Med. 101 (5): 262–4. doi:10.1258/jrsm.2008.081006. PMC 2376267. PMID 18463283.
- ↑ SMITH EL (1948). "Purification of anti-pernicious anaemia factors from liver". Nature. 161 (4095): 638. doi:10.1038/161638a0. PMID 18856623.
- ↑ Miles LM, Allen E, Clarke R, Mills K, Uauy R, Dangour AD (2017). "Impact of baseline vitamin B12 status on the effect of vitamin B12 supplementation on neurologic function in older people: secondary analysis of data from the OPEN randomised controlled trial". Eur J Clin Nutr. 71 (10): 1166–1172. doi:10.1038/ejcn.2017.7. PMID 28225050.
- ↑ Watanabe F (2007). "Vitamin B12 sources and bioavailability". Exp Biol Med (Maywood). 232 (10): 1266–74. doi:10.3181/0703-MR-67. PMID 17959839.
- ↑ Jung SB, Nagaraja V, Kapur A, Eslick GD (2015). "Association between vitamin B12 deficiency and long-term use of acid-lowering agents: a systematic review and meta-analysis". Intern Med J. 45 (4): 409–16. doi:10.1111/imj.12697. PMID 25583062.
- ↑ 6.0 6.1 Del Corral A, Carmel R (1990). "Transfer of cobalamin from the cobalamin-binding protein of egg yolk to R binder of human saliva and gastric juice". Gastroenterology. 98 (6): 1460–6. doi:10.1016/0016-5085(90)91076-i. PMID 2110915.
- ↑ Harrington DJ (2017). "Laboratory assessment of vitamin B12 status". J Clin Pathol. 70 (2): 168–173. doi:10.1136/jclinpath-2015-203502. PMID 27169753.
- ↑ Tinelli C, Di Pino A, Ficulle E, Marcelli S, Feligioni M (2019). "Hyperhomocysteinemia as a Risk Factor and Potential Nutraceutical Target for Certain Pathologies". Front Nutr. 6: 49. doi:10.3389/fnut.2019.00049. PMC 6491750. PMID 31069230.
- ↑ Callaghan JM, Khan MA, Alderuccio F, van Driel IR, Gleeson PA, Toh BH (1993). "Alpha and beta subunits of the gastric H+/K(+)-ATPase are concordantly targeted by parietal cell autoantibodies associated with autoimmune gastritis". Autoimmunity. 16 (4): 289–95. doi:10.3109/08916939309014648. PMID 7517707.
- ↑ Toh BH, Sentry JW, Alderuccio F (2000). "The causative H+/K+ ATPase antigen in the pathogenesis of autoimmune gastritis". Immunol Today. 21 (7): 348–54. doi:10.1016/s0167-5699(00)01653-4. PMID 10871877.
- ↑ Schade SG, Abels J, Schilling RF (1967). "Studies on antibody to intrinsic factor". J Clin Invest. 46 (4): 615–20. doi:10.1172/JCI105563. PMC 442045. PMID 6021209.
- ↑ Green R, Datta Mitra A (2017). "Megaloblastic Anemias: Nutritional and Other Causes". Med Clin North Am. 101 (2): 297–317. doi:10.1016/j.mcna.2016.09.013. PMID 28189172.
- ↑ Heidelbaugh JJ (2013). "Proton pump inhibitors and risk of vitamin and mineral deficiency: evidence and clinical implications". Ther Adv Drug Saf. 4 (3): 125–33. doi:10.1177/2042098613482484. PMC 4110863. PMID 25083257.
- ↑ Aroda VR, Edelstein SL, Goldberg RB, Knowler WC, Marcovina SM, Orchard TJ; et al. (2016). "Long-term Metformin Use and Vitamin B12 Deficiency in the Diabetes Prevention Program Outcomes Study". J Clin Endocrinol Metab. 101 (4): 1754–61. doi:10.1210/jc.2015-3754. PMC 4880159. PMID 26900641.
- ↑ Zulfiqar AA, Andres E (2017). "Association pernicious anemia and autoimmune polyendocrinopathy: a retrospective study". J Med Life. 10 (4): 250–253. PMC 5771255. PMID 29362601.
- ↑ Carmel R (1996). "Prevalence of undiagnosed pernicious anemia in the elderly". Arch Intern Med. 156 (10): 1097–100. PMID 8638997.
- ↑ 17.0 17.1 Andres E, Serraj K (2012). "Optimal management of pernicious anemia". J Blood Med. 3: 97–103. doi:10.2147/JBM.S25620. PMC 3441227. PMID 23028239.
- ↑ Gordon MM, Brada N, Remacha A, Badell I, del Río E, Baiget M; et al. (2004). "A genetic polymorphism in the coding region of the gastric intrinsic factor gene (GIF) is associated with congenital intrinsic factor deficiency". Hum Mutat. 23 (1): 85–91. doi:10.1002/humu.10297. PMID 14695536.
- ↑ Cattan D (2011). "Pernicious anemia: what are the actual diagnosis criteria?". World J Gastroenterol. 17 (4): 543–4. doi:10.3748/wjg.v17.i4.543. PMC 3027024. PMID 21274387.
- ↑ Seynabou F, Fatou Samba Diago N, Oulimata Diop D, Abibatou Fall S, Nafissatou D (2016). "Biermer anemia: Hematologic characteristics of 66 patients in a Clinical Hematology Unit at Senegal". Med Sante Trop. 26 (4): 402–407. doi:10.1684/mst.2016.0625. PMID 28073728.
- ↑ Han L, Wu S, Han F, Gu X (2015). "Insights into the molecular mechanisms of methylmalonic acidemia using microarray technology". Int J Clin Exp Med. 8 (6): 8866–79. PMC 4538064. PMID https://www.ncbi.nlm.nih.gov/pubmed/26309541 Check
|pmid=value (help). - ↑ Choi C, Kim T, Park KD, Lim OK, Lee JK (2019). "Subacute Combined Degeneration Caused by Nitrous Oxide Intoxication: A Report of Two Cases". Ann Rehabil Med. 43 (4): 530–534. doi:10.5535/arm.2019.43.4.530. PMC 6734019 Check
|pmc=value (help). PMID 31499607. - ↑ Stoopler ET, Kuperstein AS (2013). "Glossitis secondary to vitamin B12 deficiency anemia". CMAJ. 185 (12): E582. doi:10.1503/cmaj.120970. PMC 3761039. PMID 23359038.
- ↑ Lahner E, Annibale B (2009). "Pernicious anemia: new insights from a gastroenterological point of view". World J Gastroenterol. 15 (41): 5121–8. doi:10.3748/wjg.15.5121. PMC 2773890. PMID 19891010.
- ↑ Korman MG, Strickland RG, Hansky J (1972). "The functional 'G' cell mass in atrophic gastritis". Gut. 13 (5): 349–51. doi:10.1136/gut.13.5.349. PMC 1412218. PMID 5036089.
- ↑ "StatPearls". 2020. PMID 29939561.
- ↑ Farrelly SJ, O'Connor KA (2017). "Hypersegmented neutrophils and oval macrocytes in the setting of B12 deficiency and pancytopaenia". BMJ Case Rep. 2017. doi:10.1136/bcr-2016-218508. PMC 5612428. PMID 28821482.
- ↑ Annibale B, Lahner E, Fave GD (2011). "Diagnosis and management of pernicious anemia". Curr Gastroenterol Rep. 13 (6): 518–24. doi:10.1007/s11894-011-0225-5. PMID 21947876.
- ↑ Murphy G, Dawsey SM, Engels EA, Ricker W, Parsons R, Etemadi A; et al. (2015). "Cancer Risk After Pernicious Anemia in the US Elderly Population". Clin Gastroenterol Hepatol. 13 (13): 2282-9.e1-4. doi:10.1016/j.cgh.2015.05.040. PMC 4655146. PMID 26079040.
- ↑ Venerito M, Link A, Rokkas T, Malfertheiner P (2016). "Gastric cancer - clinical and epidemiological aspects". Helicobacter. 21 Suppl 1: 39–44. doi:10.1111/hel.12339. PMID 27531538.
- ↑ National Cancer Institute. Physician Data Query Database 2015.http://www.cancer.gov/types/leukemia/hp/cml-treatment-pdq#section/_19
- ↑ Jabbour E, Kantarjian H (May 2014). "Chronic myeloid leukemia: 2014 update on diagnosis, monitoring, and management". Am. J. Hematol. 89 (5): 547–56. doi:10.1002/ajh.23691. PMID 24729196.
- ↑ Thompson PA, Kantarjian HM, Cortes JE (October 2015). "Diagnosis and Treatment of Chronic Myeloid Leukemia in 2015". Mayo Clin. Proc. 90 (10): 1440–54. doi:10.1016/j.mayocp.2015.08.010. PMC 5656269. PMID 26434969.
- ↑ Faderl S, Talpaz M, Estrov Z, Kantarjian HM (August 1999). "Chronic myelogenous leukemia: biology and therapy". Ann. Intern. Med. 131 (3): 207–19. PMID 10428738.
- ↑ 35.0 35.1 35.2 Canadian Cancer Society.2015.http://www.cancer.ca/en/cancer-information/cancer-type/leukemia-chronic-myelogenous-cml/treatment/chronic/?region=ab
- ↑ 36.0 36.1 O'Brien SG, Guilhot F, Larson RA, Gathmann I, Baccarani M, Cervantes F, Cornelissen JJ, Fischer T, Hochhaus A, Hughes T, Lechner K, Nielsen JL, Rousselot P, Reiffers J, Saglio G, Shepherd J, Simonsson B, Gratwohl A, Goldman JM, Kantarjian H, Taylor K, Verhoef G, Bolton AE, Capdeville R, Druker BJ (March 2003). "Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia". N. Engl. J. Med. 348 (11): 994–1004. doi:10.1056/NEJMoa022457. PMID 12637609.
- ↑ Deininger MW, O'Brien SG, Ford JM, Druker BJ (April 2003). "Practical management of patients with chronic myeloid leukemia receiving imatinib". J. Clin. Oncol. 21 (8): 1637–47. doi:10.1200/JCO.2003.11.143. PMID 12668652.
- ↑ "Four Years of Follow-Up of 1027 Patients with Late Chronic Phase (L-CP), Accelerated Phase (AP), or Blast Crisis (BC) Chronic Myeloid Leukemia (CML) Treated with Imatinib in Three Large Phase II Trials. | Blood Journal".
- ↑ Kantarjian HM, Larson RA, Guilhot F, O'Brien SG, Mone M, Rudoltz M, Krahnke T, Cortes J, Druker BJ (February 2009). "Efficacy of imatinib dose escalation in patients with chronic myeloid leukemia in chronic phase". Cancer. 115 (3): 551–60. doi:10.1002/cncr.24066. PMC 4445370. PMID 19117345.
- ↑ Hochhaus A, Druker B, Sawyers C, Guilhot F, Schiffer CA, Cortes J, Niederwieser DW, Gambacorti-Passerini C, Gambacorti C, Stone RM, Goldman J, Fischer T, O'Brien SG, Reiffers JJ, Mone M, Krahnke T, Talpaz M, Kantarjian HM (February 2008). "Favorable long-term follow-up results over 6 years for response, survival, and safety with imatinib mesylate therapy in chronic-phase chronic myeloid leukemia after failure of interferon-alpha treatment". Blood. 111 (3): 1039–43. doi:10.1182/blood-2007-07-103523. PMID 17932248.
- ↑ 41.0 41.1 Druker, Brian J.; Guilhot, François; O'Brien, Stephen G.; Gathmann, Insa; Kantarjian, Hagop; Gattermann, Norbert; Deininger, Michael W.N.; Silver, Richard T.; Goldman, John M.; Stone, Richard M.; Cervantes, Francisco; Hochhaus, Andreas; Powell, Bayard L.; Gabrilove, Janice L.; Rousselot, Philippe; Reiffers, Josy; Cornelissen, Jan J.; Hughes, Timothy; Agis, Hermine; Fischer, Thomas; Verhoef, Gregor; Shepherd, John; Saglio, Giuseppe; Gratwohl, Alois; Nielsen, Johan L.; Radich, Jerald P.; Simonsson, Bengt; Taylor, Kerry; Baccarani, Michele; So, Charlene; Letvak, Laurie; Larson, Richard A. (2006). "Five-Year Follow-up of Patients Receiving Imatinib for Chronic Myeloid Leukemia". New England Journal of Medicine. 355 (23): 2408–2417. doi:10.1056/NEJMoa062867. ISSN 0028-4793.
- ↑ 42.0 42.1 Baccarani M, Saglio G, Goldman J, Hochhaus A, Simonsson B, Appelbaum F, Apperley J, Cervantes F, Cortes J, Deininger M, Gratwohl A, Guilhot F, Horowitz M, Hughes T, Kantarjian H, Larson R, Niederwieser D, Silver R, Hehlmann R (September 2006). "Evolving concepts in the management of chronic myeloid leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet". Blood. 108 (6): 1809–20. doi:10.1182/blood-2006-02-005686. PMID 16709930.
- ↑ Hochhaus A, La Rosée P (August 2004). "Imatinib therapy in chronic myelogenous leukemia: strategies to avoid and overcome resistance". Leukemia. 18 (8): 1321–31. doi:10.1038/sj.leu.2403426. PMID 15215876.
- ↑ Branford S, Rudzki Z, Walsh S, Parkinson I, Grigg A, Szer J, Taylor K, Herrmann R, Seymour JF, Arthur C, Joske D, Lynch K, Hughes T (July 2003). "Detection of BCR-ABL mutations in patients with CML treated with imatinib is virtually always accompanied by clinical resistance, and mutations in the ATP phosphate-binding loop (P-loop) are associated with a poor prognosis". Blood. 102 (1): 276–83. doi:10.1182/blood-2002-09-2896. PMID 12623848.
- ↑ Forrest DL, Jiang X, Eaves CJ, Smith CL (April 2008). "An approach to the management of chronic myeloid leukemia in British Columbia". Curr Oncol. 15 (2): 90–7. PMC 2365478. PMID 18454182.
- ↑ Soverini S, Martinelli G, Rosti G, Bassi S, Amabile M, Poerio A, Giannini B, Trabacchi E, Castagnetti F, Testoni N, Luatti S, de Vivo A, Cilloni D, Izzo B, Fava M, Abruzzese E, Alberti D, Pane F, Saglio G, Baccarani M (June 2005). "ABL mutations in late chronic phase chronic myeloid leukemia patients with up-front cytogenetic resistance to imatinib are associated with a greater likelihood of progression to blast crisis and shorter survival: a study by the GIMEMA Working Party on Chronic Myeloid Leukemia". J. Clin. Oncol. 23 (18): 4100–9. doi:10.1200/JCO.2005.05.531. PMID 15867198.
- ↑ Gorre, M. E. (2001). "Clinical Resistance to STI-571 Cancer Therapy Caused by BCR-ABL Gene Mutation or Amplification". Science. 293 (5531): 876–880. doi:10.1126/science.1062538. ISSN 0036-8075.
- ↑ Mahon FX, Deininger MW, Schultheis B, Chabrol J, Reiffers J, Goldman JM, Melo JV (August 2000). "Selection and characterization of BCR-ABL positive cell lines with differential sensitivity to the tyrosine kinase inhibitor STI571: diverse mechanisms of resistance". Blood. 96 (3): 1070–9. PMID 10910924.
- ↑ Raz A, Kloog Y, Perel E, Kenig-Wakshal R, Bose KS, Sarma RH, Fowler NO, McCall D, Chou TC, Holmes JC, Hanenson IB (September 1975). "Properties of solubilized prostaglandin synthetase from sheep vesicular glands". Life Sci. 17 (6): 951–8. PMID 571.
- ↑ Egan DN, Beppu L, Radich JP (January 2015). "Patients with Philadelphia-positive leukemia with BCR-ABL kinase mutations before allogeneic transplantation predominantly relapse with the same mutation". Biol. Blood Marrow Transplant. 21 (1): 184–9. doi:10.1016/j.bbmt.2014.09.012. PMC 4464836. PMID 25300870.
- ↑ Kantarjian H, O'Brien S, Cortes J, Giles F, Shan J, Rios MB, Faderl S, Verstovsek S, Garcia-Manero G, Wierda W, Kornblau S, Ferrajoli A, Keating M, Talpaz M (January 2004). "Survival advantage with imatinib mesylate therapy in chronic-phase chronic myelogenous ;eukemia (CML-CP) after IFN-alpha failure and in late CML-CP, comparison with historical controls". Clin. Cancer Res. 10 (1 Pt 1): 68–75. PMID 14734453.
- ↑ Kantarjian H, Sawyers C, Hochhaus A, Guilhot F, Schiffer C, Gambacorti-Passerini C, Niederwieser D, Resta D, Capdeville R, Zoellner U, Talpaz M, Druker B, Goldman J, O'Brien SG, Russell N, Fischer T, Ottmann O, Cony-Makhoul P, Facon T, Stone R, Miller C, Tallman M, Brown R, Schuster M, Loughran T, Gratwohl A, Mandelli F, Saglio G, Lazzarino M, Russo D, Baccarani M, Morra E (February 2002). "Hematologic and cytogenetic responses to imatinib mesylate in chronic myelogenous leukemia". N. Engl. J. Med. 346 (9): 645–52. doi:10.1056/NEJMoa011573. PMID 11870241.
- ↑ Hughes TP, Kaeda J, Branford S, Rudzki Z, Hochhaus A, Hensley ML, Gathmann I, Bolton AE, van Hoomissen IC, Goldman JM, Radich JP (October 2003). "Frequency of major molecular responses to imatinib or interferon alfa plus cytarabine in newly diagnosed chronic myeloid leukemia". N. Engl. J. Med. 349 (15): 1423–32. doi:10.1056/NEJMoa030513. PMID 14534335.
- ↑ Roy L, Guilhot J, Krahnke T, Guerci-Bresler A, Druker BJ, Larson RA, O'Brien S, So C, Massimini G, Guilhot F (September 2006). "Survival advantage from imatinib compared with the combination interferon-alpha plus cytarabine in chronic-phase chronic myelogenous leukemia: historical comparison between two phase 3 trials". Blood. 108 (5): 1478–84. doi:10.1182/blood-2006-02-001495. PMID 16627756.
- ↑ Kantarjian HM, Talpaz M, O'Brien S, Jones D, Giles F, Garcia-Manero G, Faderl S, Ravandi F, Rios MB, Shan J, Cortes J (September 2006). "Survival benefit with imatinib mesylate versus interferon-alpha-based regimens in newly diagnosed chronic-phase chronic myelogenous leukemia". Blood. 108 (6): 1835–40. doi:10.1182/blood-2006-02-004325. PMID 16709931.
- ↑ Cortes J, Talpaz M, O'Brien S, Jones D, Luthra R, Shan J, Giles F, Faderl S, Verstovsek S, Garcia-Manero G, Rios MB, Kantarjian H (May 2005). "Molecular responses in patients with chronic myelogenous leukemia in chronic phase treated with imatinib mesylate". Clin. Cancer Res. 11 (9): 3425–32. doi:10.1158/1078-0432.CCR-04-2139. PMID 15867244.
- ↑ Weisberg E, Manley PW, Breitenstein W, Brüggen J, Cowan-Jacob SW, Ray A, Huntly B, Fabbro D, Fendrich G, Hall-Meyers E, Kung AL, Mestan J, Daley GQ, Callahan L, Catley L, Cavazza C, Azam M, Mohammed A, Neuberg D, Wright RD, Gilliland DG, Griffin JD (February 2005). "Characterization of AMN107, a selective inhibitor of native and mutant Bcr-Abl". Cancer Cell. 7 (2): 129–41. doi:10.1016/j.ccr.2005.01.007. PMID 15710326.
- ↑ Saglio G, Kim DW, Issaragrisil S, le Coutre P, Etienne G, Lobo C, Pasquini R, Clark RE, Hochhaus A, Hughes TP, Gallagher N, Hoenekopp A, Dong M, Haque A, Larson RA, Kantarjian HM (June 2010). "Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia". N. Engl. J. Med. 362 (24): 2251–9. doi:10.1056/NEJMoa0912614. PMID 20525993.
- ↑ Brümmendorf TH, Cortes JE, de Souza CA, Guilhot F, Duvillié L, Pavlov D, Gogat K, Countouriotis AM, Gambacorti-Passerini C (January 2015). "Bosutinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukaemia: results from the 24-month follow-up of the BELA trial". Br. J. Haematol. 168 (1): 69–81. doi:10.1111/bjh.13108. PMC 4274978. PMID 25196702.
- ↑ 60.0 60.1 Cortes JE, Gambacorti-Passerini C, Deininger MW, Mauro MJ, Chuah C, Kim DW, Dyagil I, Glushko N, Milojkovic D, le Coutre P, Garcia-Gutierrez V, Reilly L, Jeynes-Ellis A, Leip E, Bardy-Bouxin N, Hochhaus A, Brümmendorf TH (January 2018). "Bosutinib Versus Imatinib for Newly Diagnosed Chronic Myeloid Leukemia: Results From the Randomized BFORE Trial". J. Clin. Oncol. 36 (3): 231–237. doi:10.1200/JCO.2017.74.7162. PMC 5966023. PMID 29091516.
- ↑ Parker WT, Yeung DT, Yeoman AL, Altamura HK, Jamison BA, Field CR; et al. (2016). "The impact of multiple low-level BCR-ABL1 mutations on response to ponatinib". Blood. 127 (15): 1870–80. doi:10.1182/blood-2015-09-666214. PMC 4832506. PMID 26773037.
- ↑ Gambacorti-Passerini C, Antolini L, Mahon FX, Guilhot F, Deininger M, Fava C, Nagler A, Della Casa CM, Morra E, Abruzzese E, D'Emilio A, Stagno F, le Coutre P, Hurtado-Monroy R, Santini V, Martino B, Pane F, Piccin A, Giraldo P, Assouline S, Durosinmi MA, Leeksma O, Pogliani EM, Puttini M, Jang E, Reiffers J, Piazza R, Valsecchi MG, Kim DW (April 2011). "Multicenter independent assessment of outcomes in chronic myeloid leukemia patients treated with imatinib". J. Natl. Cancer Inst. 103 (7): 553–61. doi:10.1093/jnci/djr060. PMID 21422402.
- ↑ Aichberger KJ, Herndlhofer S, Schernthaner GH, Schillinger M, Mitterbauer-Hohendanner G, Sillaber C, Valent P (July 2011). "Progressive peripheral arterial occlusive disease and other vascular events during nilotinib therapy in CML". Am. J. Hematol. 86 (7): 533–9. doi:10.1002/ajh.22037. PMID 21538470.
- ↑ Yeung DT, Osborn MP, White DL, Branford S, Braley J, Herschtal A, Kornhauser M, Issa S, Hiwase DK, Hertzberg M, Schwarer AP, Filshie R, Arthur CK, Kwan YL, Trotman J, Forsyth CJ, Taper J, Ross DM, Beresford J, Tam C, Mills AK, Grigg AP, Hughes TP (February 2015). "TIDEL-II: first-line use of imatinib in CML with early switch to nilotinib for failure to achieve time-dependent molecular targets". Blood. 125 (6): 915–23. doi:10.1182/blood-2014-07-590315. PMC 5161008. PMID 25519749.
- ↑ "The price of drugs for chronic myeloid leukemia (CML) is a reflection of the unsustainable prices of cancer drugs: from the perspective of a large group of CML experts". Blood. 121 (22): 4439–42. May 2013. doi:10.1182/blood-2013-03-490003. PMC 4190613. PMID 23620577.
- ↑ Dusetzina SB, Winn AN, Abel GA, Huskamp HA, Keating NL (February 2014). "Cost sharing and adherence to tyrosine kinase inhibitors for patients with chronic myeloid leukemia". J. Clin. Oncol. 32 (4): 306–11. doi:10.1200/JCO.2013.52.9123. PMID 24366936.
- ↑ Gratwohl A, Hermans J, Goldman JM, Arcese W, Carreras E, Devergie A, Frassoni F, Gahrton G, Kolb HJ, Niederwieser D, Ruutu T, Vernant JP, de Witte T, Apperley J (October 1998). "Risk assessment for patients with chronic myeloid leukaemia before allogeneic blood or marrow transplantation. Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation". Lancet. 352 (9134): 1087–92. PMID 9798583.
- ↑ Saussele S, Lauseker M, Gratwohl A, Beelen DW, Bunjes D, Schwerdtfeger R, Kolb HJ, Ho AD, Falge C, Holler E, Schlimok G, Zander AR, Arnold R, Kanz L, Dengler R, Haferlach C, Schlegelberger B, Pfirrmann M, Müller MC, Schnittger S, Leitner A, Pletsch N, Hochhaus A, Hasford J, Hehlmann R (March 2010). "Allogeneic hematopoietic stem cell transplantation (allo SCT) for chronic myeloid leukemia in the imatinib era: evaluation of its impact within a subgroup of the randomized German CML Study IV". Blood. 115 (10): 1880–5. doi:10.1182/blood-2009-08-237115. PMID 19965667.
- ↑ Baccarani M, Deininger MW, Rosti G, Hochhaus A, Soverini S, Apperley JF, Cervantes F, Clark RE, Cortes JE, Guilhot F, Hjorth-Hansen H, Hughes TP, Kantarjian HM, Kim DW, Larson RA, Lipton JH, Mahon FX, Martinelli G, Mayer J, Müller MC, Niederwieser D, Pane F, Radich JP, Rousselot P, Saglio G, Saußele S, Schiffer C, Silver R, Simonsson B, Steegmann JL, Goldman JM, Hehlmann R (August 2013). "European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013". Blood. 122 (6): 872–84. doi:10.1182/blood-2013-05-501569. PMC 4915804. PMID 23803709.
- ↑ Apperley JF (July 2013). "TWIST it but don't spin it". Blood. 122 (4): 470–1. doi:10.1182/blood-2013-06-506097. PMID 23886774.
- ↑ Michor F, Hughes TP, Iwasa Y, Branford S, Shah NP, Sawyers CL, Nowak MA (June 2005). "Dynamics of chronic myeloid leukaemia". Nature. 435 (7046): 1267–70. doi:10.1038/nature03669. PMID 15988530.
- ↑ Angstreich GR, Matsui W, Huff CA, Vala MS, Barber J, Hawkins AL, Griffin CA, Smith BD, Jones RJ (August 2005). "Effects of imatinib and interferon on primitive chronic myeloid leukaemia progenitors". Br. J. Haematol. 130 (3): 373–81. doi:10.1111/j.1365-2141.2005.05606.x. PMID 16042686.