Tuberculosis laboratory findings: Difference between revisions
Mohamed riad (talk | contribs) |
Mohamed riad (talk | contribs) |
||
| Line 9: | Line 9: | ||
Routine laboratory tests are usually normal. In some patients, the subsequent abnormalities could also be found: | Routine laboratory tests are usually normal. In some patients, the subsequent abnormalities could also be found: | ||
*Mild normocytic [[anemia]] | *Mild normocytic [[anemia]] can be present in almost half of patients. | ||
*Increased [[erythrocyte sedimentation rate]] (ESR) | *Increased [[erythrocyte sedimentation rate]] (ESR) | ||
*Mild [[leukocytosis]] | *Mild [[leukocytosis]] | ||
| Line 16: | Line 16: | ||
*Elevated [[alkaline phosphatase]] levels | *Elevated [[alkaline phosphatase]] levels | ||
*Mild elevation of [[liver enzymes]] | *Mild elevation of [[liver enzymes]] | ||
*Findings of [[adrenal insufficiency]] in case of adrenal involvement (low [[cortisol]] and [[aldosterone]] levels) | *Findings of [[adrenal insufficiency]] in case of [[adrenal gland]] involvement (low [[cortisol]] and [[aldosterone]] levels) | ||
===Acid-Fast Bacilli=== | ===Acid-Fast Bacilli=== | ||
| Line 34: | Line 34: | ||
A positive [[Culture medium|culture]] for [[Mycobacterium tuberculosis|M. tuberculosis]] confirms the diagnosis of TB disease. [[Culture medium|Culture]] examinations are crucial and should be done on all [[specimens]], regardless of [[AFB smear]] results. Laboratories are necessary to report positive results on smears and [[cultures]] within 24 hours by telephone or fax to the primary health care provider and to the state or local [[Tuberculosis|TB]] control program, as needed by law. | A positive [[Culture medium|culture]] for [[Mycobacterium tuberculosis|M. tuberculosis]] confirms the diagnosis of TB disease. [[Culture medium|Culture]] examinations are crucial and should be done on all [[specimens]], regardless of [[AFB smear]] results. Laboratories are necessary to report positive results on smears and [[cultures]] within 24 hours by telephone or fax to the primary health care provider and to the state or local [[Tuberculosis|TB]] control program, as needed by law. | ||
Many types of [[cultures]] are available for [[Mycobacterium tuberculosis|M. tuberculosis]], including [[Löwenstein-Jensen]] (LJ), [[Kirchner]], or Middlebrook media. <ref name="pmid12614730">{{cite journal |author=Drobniewski F, Caws M, Gibson A, Young D |title=Modern laboratory diagnosis of tuberculosis |journal=Lancet Infect Dis |volume=3 |issue=3 |pages=141-7 |year=2003 |id=PMID 12614730}}</ref>. [[Culture medium|Culture]] of [[Mycobacterium|Mycobacteria]] often takes 4--8 weeks to grow and can help distinguish from several types of [[Mycobacterium|mycobacteria]]. Other | Many types of [[cultures]] are available for [[Mycobacterium tuberculosis|M. tuberculosis]], including [[Löwenstein-Jensen]] (LJ), [[Kirchner]], or Middlebrook media. <ref name="pmid12614730">{{cite journal |author=Drobniewski F, Caws M, Gibson A, Young D |title=Modern laboratory diagnosis of tuberculosis |journal=Lancet Infect Dis |volume=3 |issue=3 |pages=141-7 |year=2003 |id=PMID 12614730}}</ref>. [[Culture medium|Culture]] of [[Mycobacterium|Mycobacteria]] often takes 4--8 weeks to grow and can help distinguish from several types of [[Mycobacterium|mycobacteria]]. Other kinds of [[Culture medium|culture]] are available, this include automated systems in which the [[Mycobacterium|mycobacteria]] grow at a faster rate. [[MB/BacT]], BACTEC 9000, as well as the [[Mycobacterial Growth Indicator Tube]] (MGIT). The microscopic-observation drug-susceptibility (MODS) culture is considered a faster and more accurate method <ref name="pmid17035648">{{cite journal |author=Moore D, Evans C, Gilman R, Caviedes L, Coronel J, Vivar A, Sanchez E, Piñedo Y, Saravia J, Salazar C, Oberhelman R, Hollm-Delgado M, LaChira D, Escombe A, Friedland J |title=Microscopic-observation drug-susceptibility assay for the diagnosis of TB |journal=N Engl J Med |volume=355 |issue=15 |pages=1539-50 |year=2006 |id=PMID 17035648}}</ref><ref name="MinionLeung2010">{{cite journal|last1=Minion|first1=Jessica|last2=Leung|first2=Erika|last3=Menzies|first3=Dick|last4=Pai|first4=Madhukar|title=Microscopic-observation drug susceptibility and thin layer agar assays for the detection of drug resistant tuberculosis: a systematic review and meta-analysis|journal=The Lancet Infectious Diseases|volume=10|issue=10|year=2010|pages=688–698|issn=14733099|doi=10.1016/S1473-3099(10)70165-1}}</ref>. | ||
{| | {| | ||
Revision as of 07:10, 26 March 2021
|
Tuberculosis Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Tuberculosis laboratory findings On the Web |
|
American Roentgen Ray Society Images of Tuberculosis laboratory findings |
|
Risk calculators and risk factors for Tuberculosis laboratory findings |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Mashal Awais, M.D.[2]; Alejandro Lemor, M.D. [3]
Overview
Routine laboratory exams are usually normal. The presence of acid-fast-bacilli (AFB) on a sputum smear or another specimen usually indicates TB disease and a positive culture for M. tuberculosis confirms the diagnosis. Other examples of laboratory tests are urinalysis, peritoneal fluid, or CSF analysis and Interferon-Gamma release assays (IGRA).
Laboratory Findings
Routine laboratory tests are usually normal. In some patients, the subsequent abnormalities could also be found:
- Mild normocytic anemia can be present in almost half of patients.
- Increased erythrocyte sedimentation rate (ESR)
- Mild leukocytosis
- Hyponatremia
- Hypercalcemia
- Elevated alkaline phosphatase levels
- Mild elevation of liver enzymes
- Findings of adrenal insufficiency in case of adrenal gland involvement (low cortisol and aldosterone levels)
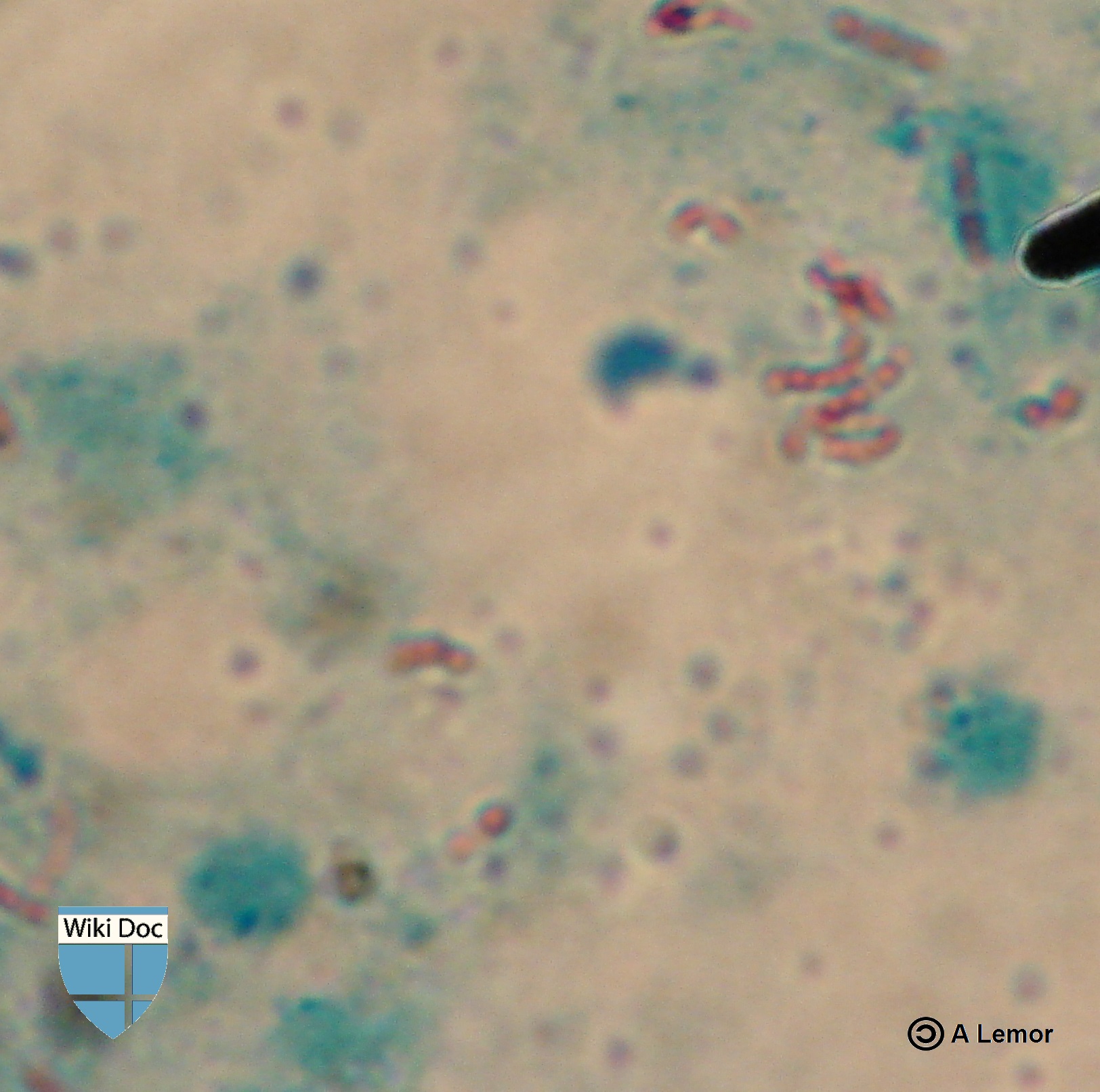
Acid-Fast Bacilli
Presence of acid-fast-bacilli (AFB) on sputum smear or on another specimen usually indicates TB disease. Although acid-fast microscopy is easy and quick, it does not confirm a diagnosis of Tuberculosis as some acid-fast-bacilli are not M. tuberculosis. Consequently, a culture is done on all initial samples for confirmation of the diagnosis.
Sputum smears and cultures should be performed to detect acid-fast bacilli if the patient is producing sputum. The best method for this is fluorescence microscopy (auramine-rhodamine staining), which is more sensitive than conventional Ziehl-Neelsen staining.[1]
In case of inability of sufficient sputum production, specimens can be obtained by inducing sputum, gastric washings, a laryngeal swab, bronchoscopy with bronchoalveolar lavage, or fine-needle aspiration of a collection. A comparative study reported that inducing three sputum samples is more sensitive than three gastric washings.[2]
Other mycobacteria are also acid-fast. If the smear is positive, PCR or gene probe testing can differentiate M. tuberculosis from other mycobacteria. Even if sputum smear is negative, tuberculosis cannot be excluded without obtaining negative cultures.
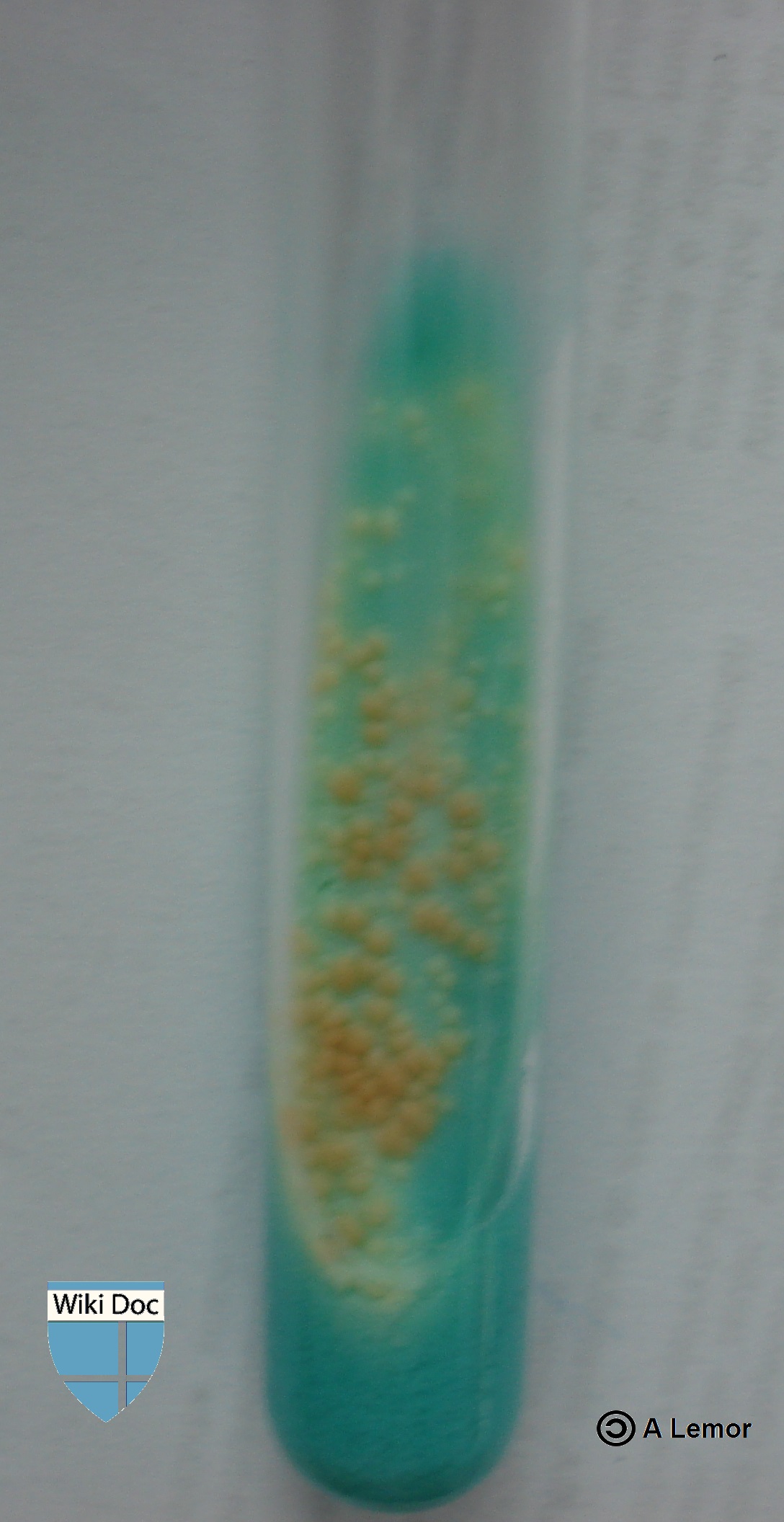
Culture
A positive culture for M. tuberculosis confirms the diagnosis of TB disease. Culture examinations are crucial and should be done on all specimens, regardless of AFB smear results. Laboratories are necessary to report positive results on smears and cultures within 24 hours by telephone or fax to the primary health care provider and to the state or local TB control program, as needed by law.
Many types of cultures are available for M. tuberculosis, including Löwenstein-Jensen (LJ), Kirchner, or Middlebrook media. [3]. Culture of Mycobacteria often takes 4--8 weeks to grow and can help distinguish from several types of mycobacteria. Other kinds of culture are available, this include automated systems in which the mycobacteria grow at a faster rate. MB/BacT, BACTEC 9000, as well as the Mycobacterial Growth Indicator Tube (MGIT). The microscopic-observation drug-susceptibility (MODS) culture is considered a faster and more accurate method [4][5].
 |
 |
A sample of pleural exudate can be analyzed by cytopathology or at a cell count lab. Samples are usually lymphocyte predominant, and cytopathology is more accurate than cell count labs at detecting lymph. If there is more fluid present, then an AFB lab is more appropriate. A pleural exudate lab test may find sterile pyuria (especially in HIV positive patients), but overall this finding is fairly uncommon. Most extra-pulmonary TB is paucibacillary, so the yield of tests is very low. A negative result does not exclude a tuberculosis infection.
Drug Resistance
For all patients, the initial M. tuberculosis isolate should be tested for drug resistance. It is important to identify drug resistance as early as possible to ensure effective treatment. Drug susceptibility testing should be repeated for patients who do not respond adequately to treatment or who have positive culture results despite 3 months of therapy. Susceptibility results from laboratories should be reported to the primary health care provider and the state or local TB control programs.
Fluid Analysis
- Peritoneal Fluid: Leucocyte count: 150-4000 mm³, lymphocytic pleocytosis, protein > 3.0 g/dL, increased LDH.
- Cerebrospinal Fluid: High protein, low glucose, increased number of lymphocytes, increased LDH
Urinalysis
- For renal tuberculosis, the classic laboratory finding is sterile pyuria with microscopic hematuria.
Interferon-Gamma Release Assays (IGRAs)
Interferon-Gamma Release Assays (IGRAs) are whole-blood tests that help in diagnosing Mycobacterium tuberculosis infection, but they do not differentiate latent tuberculosis infection (LTBI) from tuberculosis disease. Two IGRAs tests that are approved by the U.S. Food and Drug Administration (FDA) and commercially available in the U.S are The QuantiFERON®-TB Gold In-Tube test (QFT-GIT) and the T-SPOT®.TB test (T-Spot).
IGRAs measure a person’s immune reactivity to M. tuberculosis antigen. White blood cells from most persons that have been infected with M. tuberculosis will release interferon-gamma (IFN-g) when mixed with antigens (substances that can produce an immune response) derived from M. tuberculosis.
To perform this test, fresh blood samples are mixed with antigens and controls. The antigens, testing methods, and interpretation criteria for IGRAs differ.
| QuantiFERON | T-SPOT | |
|---|---|---|
| Initial Process | Process whole blood within 16 hours | Process peripheral blood mononuclear cells (PBMCs) within 8 hours, or if T-Cell Xtend® is used, within 30 hours |
| M. tuberculosis Antigen | Single mixture of synthetic peptides representing ESAT-6, CFP-10 & TB7.7. | Separate mixtures of synthetic peptides representing ESAT-6 & CFP-10 |
| Measurement | IFN-g concentration | Number of IFN-g producing cells (spots) |
| Possible Results | Positive, negative, indeterminate | Positive, negative, indeterminate, borderline |
| Table adapted from CDC [6] | ||
| Advantages | Disadvantages |
|---|---|
|
|
| Table adapted from CDC Interferon-Gamma Release Assays[6] | |
CDC Recommendations on When to Use IGRA Tests Adapted from CDC Interferon-Gamma Release Assays[6]
- IGRAs can also be used in place of (but not in addition to) TST in all situations in which CDC recommends TST as an aid in diagnosing M. tuberculosis infection, with preferences and special considerations noted below.
- This includes contact investigations, testing during pregnancy, and screening of health care workers, and others undergoing serial evaluation for M. tuberculosis infection.
- Despite the indication of a preference, the use of the alternative test (FDA-approved IGRA or TST) is acceptable medical and public health practice.
- We should be cautious in interpretation when testing certain populations because of limited data on the use of IGRAs
- Populations in which IGRAs are preferred for testing:
- Persons who have received BCG (either as a vaccine or for cancer therapy)
- Persons from groups that historically have less chances of return for TST reading.
- TST is preferred over IGRAs for children less than 5 years of age.
- As with TST, IGRAs generally should not be used for testing persons who have a low risk of infection and a low risk of disease due to M. tuberculosis.
- Each institution and TB control program should evaluate the availability and benefits of IGRAs in prioritizing their use.
Routine testing with both TST and IGRA is not recommended. However, results from both tests might be useful in the following situations:
| When the initial test is negative and: | When the initial test is positive and: |
|---|---|
|
|
| Table adapted from CDC[6] | |
- In addition, repeating an IGRA or performing a TST might be useful when the initial IGRA result is indeterminate, borderline, or invalid and a reason for testing persists.
- Multiple negative results from any combination of these tests cannot exclude M. tuberculosis infection. Steps should be taken to minimize unnecessary and misleading testing of persons at low risk.
- Selection of the most suitable test or combination of tests for detection of M. tuberculosis infection should be based on the reasons and the context for testing, test availability, and overall cost of testing.
References
- ↑ Steingart K, Henry M, Ng V; et al. (2006). "Fluorescence versus conventional sputum smear microscopy for tuberculosis: a systematic review". Lancet Infect Dis. 6 (9): 570&ndash, 81. doi:10.1016/S1473-3099(06)70578-3.
- ↑ Brown M, Varia H, Bassett P, Davidson RN, Wall R, Pasvol G (2007). "Prospective study of sputum induction, gastric washing, and bronchoalveolar lavage for the diagnosis of pulmonary tuberculosis in patients who are unable to expectorate". Clin Infect Dis. 44 (11): 1415–20. doi:10.1086/516782. PMID 17479935.
- ↑ Drobniewski F, Caws M, Gibson A, Young D (2003). "Modern laboratory diagnosis of tuberculosis". Lancet Infect Dis. 3 (3): 141–7. PMID 12614730.
- ↑ Moore D, Evans C, Gilman R, Caviedes L, Coronel J, Vivar A, Sanchez E, Piñedo Y, Saravia J, Salazar C, Oberhelman R, Hollm-Delgado M, LaChira D, Escombe A, Friedland J (2006). "Microscopic-observation drug-susceptibility assay for the diagnosis of TB". N Engl J Med. 355 (15): 1539–50. PMID 17035648.
- ↑ Minion, Jessica; Leung, Erika; Menzies, Dick; Pai, Madhukar (2010). "Microscopic-observation drug susceptibility and thin layer agar assays for the detection of drug resistant tuberculosis: a systematic review and meta-analysis". The Lancet Infectious Diseases. 10 (10): 688–698. doi:10.1016/S1473-3099(10)70165-1. ISSN 1473-3099.
- ↑ 6.0 6.1 6.2 6.3 "CDC Interferon-Gamma Release Assays (IGRAs) - Blood Tests for TB Infection".