Trichomoniasis
| Trichomoniasis | |
 | |
|---|---|
| Trichomoniasis epidemiology | |
| ICD-10 | A59 |
| ICD-9 | 131 |
| DiseasesDB | 13334 |
| eMedicine | med/2308 emerg/613 |
| MeSH | D014246 |
|
WikiDoc Resources for Trichomoniasis |
|
Articles |
|---|
|
Most recent articles on Trichomoniasis Most cited articles on Trichomoniasis |
|
Media |
|
Powerpoint slides on Trichomoniasis |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Trichomoniasis at Clinical Trials.gov Trial results on Trichomoniasis Clinical Trials on Trichomoniasis at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Trichomoniasis NICE Guidance on Trichomoniasis
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Trichomoniasis Discussion groups on Trichomoniasis Patient Handouts on Trichomoniasis Directions to Hospitals Treating Trichomoniasis Risk calculators and risk factors for Trichomoniasis
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Trichomoniasis |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Please Take Over This Page and Apply to be Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [2] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
Overview
Trichomoniasis also known as Trichomonas Infection [3] sometimes referred to as "trich", is a common sexually transmitted disease that affects 7.4 million previously unaffected Americans each year. It is caused by a single-celled protozoan parasite Trichomonas vaginalis. Trichomoniasis is primarily an infection of the genitourinary tract; The urethra is the most common site of infection in men, and the vagina is the most common site of infection in women. It is most common in women and uncircumsized men. For uncircumsized men, the most common site for the infection is the tip of the penis.
Epidemiology and Demographics
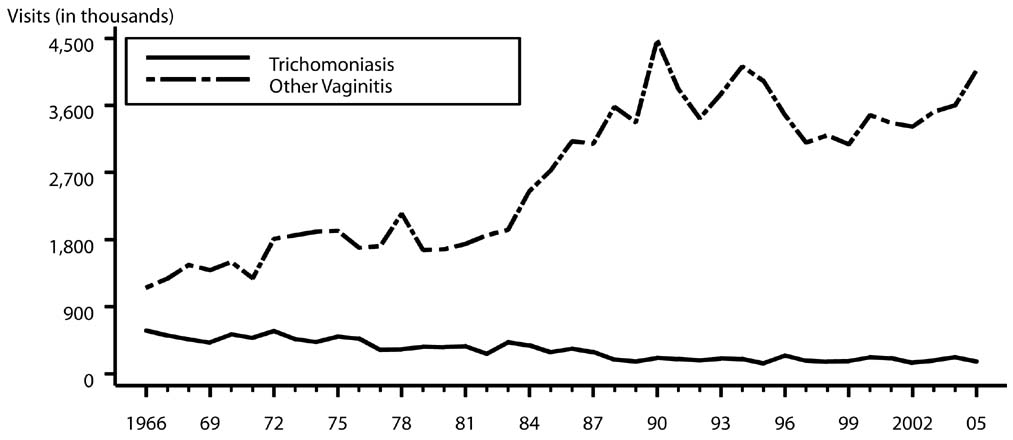
Trichomoniasis is the most common curable STD in young, sexually active women. An estimated 7.4 million new cases occur each year in women and men. The relative standard error for trichomoniasis estimates range from 7.5% to 13% and for other vaginitis estimates range from 16% to 30%.
Trichomonas vaginalis, a flagellate, is the most common pathogenic protozoan of humans in industrialized countries. However, it occurs worldwide.
Trichomoniasis and other vaginal infections in women — Initial visits to physicians' offices: United States, 1966–2005:
Risk Factors
Higher prevalence among persons with multiple sexual partners or other venereal diseases.[7]
Pathophysiology & Etiology
Trichomoniasis is caused by the single-celled protozoan parasite, Trichomonas vaginalis. The vagina is the most common site of infection in women, and the urethra (urine canal) is the most common site of infection in men. The parasite is sexually transmitted through penis-to-vagina intercourse or vulva-to-vulva (the genital area outside the vagina) contact with an infected partner. Women can acquire the disease from infected men or women, but men usually contract it only from infected women.
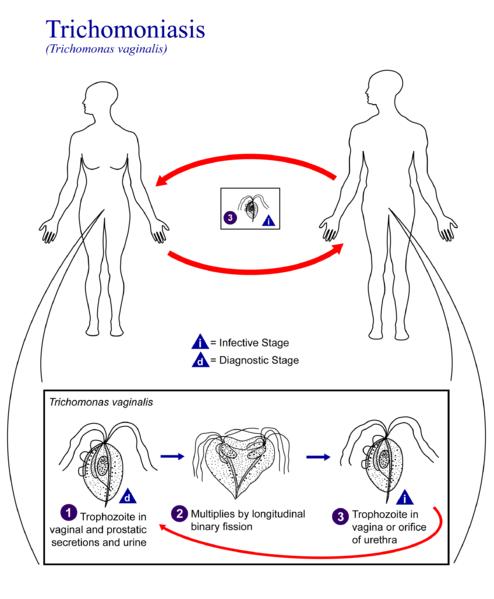
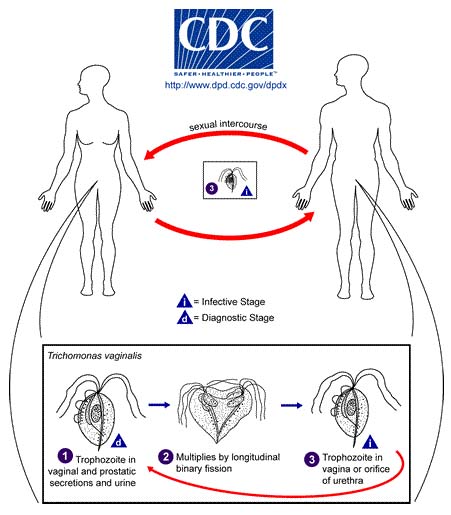
Life Cycle Trichomonas vaginalis resides in the female lower genital tract and the male urethra and prostate 1, where it replicates by binary fission 2. The parasite does not appear to have a cyst form, and does not survive well in the external environment. Trichomonas vaginalis is transmitted among humans, its only known host, primarily by sexual intercourse 3.
Molecular Biology
A draft sequence of the Trichomoniasis genome was published on January 12, 2007 in the journal Science confirming that the genome has at least 26,000 genes, a similar number to the human genome.[10]
Diagnosis
For both men and women, a health care provider must perform a physical examination and laboratory test to diagnose trichomoniasis. The parasite is harder to detect in men than in women. In women, a pelvic examination can reveal small red ulcerations (sores) on the vaginal wall or cervix.
Diagnosis of vaginal trichomoniasis is usually performed by microscopy of vaginal secretions, but this method has a sensitivity of only approximately 60%–70% and requires immediate evaluation of wet preparation slide for optimal results. Other FDA-cleared tests for trichomoniasis in women include OSOM Trichomonas Rapid Test (Genzyme Diagnostics, Cambridge, Massachusetts), an immunochromatographic capillary flow dipstick technology, and the Affirm™ VP III (Becton Dickenson, San Jose, California), a nucleic acid probe test that evaluates for T. vaginalis, G. vaginalis, and C. albicans. These tests are both performed on vaginal secretions and have a sensitivity >83% and a specificity >97%. Both tests are point-of-care diagnostics. The results of the OSOM Trichomonas Rapid Test are available in approximately 10 minutes, and results of the Affirm™ VP III are available within 45 minutes. Although these tests tend to be more sensitive than vaginal wet preparation, false positives might occur especially in low prevalence populations. Culture is the most sensitive and specific commercially available method of diagnosis. In women in whom trichomoniasis is suspected but not confirmed by microscopy, vaginal secretions should be cultured for T. vaginalis.
In men, wet preparation is insensitive, and culture testing of urethral swab, urine, and semen is required for optimal sensitivity. No FDA-cleared PCR test for T. vaginalis is available in the United States, but such testing might be available from commercial laboratories that have developed their own PCR tests. [11] [12]
History and Symptoms
Trichomoniasis, like many other sexually transmitted diseases, often occurs without any symptoms. Men almost never have symptoms, while 20% of women are asymptomatic.
Some men may temporarily have an irritation inside the penis, a thin, whitish discharge from the penis and painful or difficult urination.
When women have symptoms, they usually appear within 5 to 28 days of exposure. The symptoms in women include a heavy, yellow-green or gray vaginal discharge, discomfort during intercourse, unpleasant vaginal odor, and painful urination. Irritation and itching of the female genital area, and on rare occasions, lower abdominal pain also can be present. In about two-thirds of infected females, there is edema, inflammation, cell hypertrophy and metaplasia. [13]
Laboratory Findings
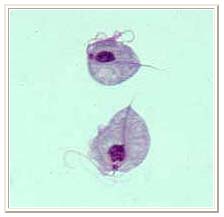
Two trophozoites of Trichomonas vaginalis obtained from in vitro culture. Smear was stained with Giemsa.[14]
Risk Stratification and Prognosis
- The genital inflammation caused by trichomoniasis can increase a woman's susceptibility to HIV infection if she is exposed to the virus. Having trichomoniasis may increase the chance that an HIV-infected woman passes HIV to her sex partner(s).
- Pregnant women with trichomoniasis may have babies who are born early or with low birth weight (less than five pounds).[15]
Research has shown a link between trichomoniasis and two serious sequelæ. Data suggest that:
- Trichomoniasis is associated with increased risk of transmission of HIV.
- Trichomoniasis may cause a woman to deliver a low-birth-weight or premature infant.
Additional research is needed to fully explore these relationships.
Treatment
Trichomoniasis can usually be cured with the prescription drug, metronidazole, given by mouth in a single dose. The symptoms of trichomoniasis in infected men may disappear within a few weeks without treatment. However, an infected man, even a man who has never had symptoms or whose symptoms have stopped, can continue to infect or re-infect a female partner until he has been treated. Therefore, both partners should be treated at the same time to eliminate the parasite. Persons being treated for trichomoniasis should avoid sex until they and their sex partners complete treatment and have no symptoms. Metronidazole can be used by pregnant women.
Having trichomoniasis once does not protect a person from getting it again. Following successful treatment, people can still be susceptible to re-infection.
Pharmacotherapy
Acute Pharmacotherapies
- Metronidazole: 2 g orally in a single dose, or
- Tinidazole: 2 g orally in a single dose
- Alternative Regimen: Metronidazole 500 mg orally twice a day for 7 days
Patients should be advised to avoid consuming alcohol during treatment with metronidazole or tinidazole. Abstinence from alcohol use should continue for 24 hours after completion of metronidazole or 72 hours after completion of tinidazole.
The nitroimidazoles comprise the only class of drugs useful for the oral or parenteral therapy of trichomoniasis. Of these drugs, metronidazole and tinidazole are available in the United States and are cleared by the FDA for the treatment of trichomoniasis. In randomized clinical trials, the recommended metronidazole regimens have resulted in cure rates of approximately 90%–95%, and the recommended tinidazole regimen has resulted in cure rates of approximately 86%–100%. The appropriate treatment of sex partners might increase these reported rates. Randomized controlled trials comparing single 2 g doses of metronidazole and tinidazole suggest that tinidazole is equivalent to, or superior to, metronidazole in achieving parasitologic cure and resolution of symptoms (170). Treatment of patients and sex partners results in relief of symptoms, microbiologic cure, and reduction of transmission.
Metronidazole gel is considerably less efficacious for the treatment of trichomoniasis (<50%) than oral preparations of metronidazole. Topically applied antimicrobials (e.g., metronidazole gel) are unlikely to achieve therapeutic levels in the urethra or perivaginal glands; therefore, use of the gel is not recommended. Several other topically applied antimicrobials occasionally have been used for treatment of trichomoniasis; however, these preparations probably do not have greater efficacy than metronidazole gel.
Follow-Up:
Follow-up is unnecessary for men and women who become asymptomatic after treatment or who are initially asymptomatic. Some strains of T. vaginalis can have diminished susceptibility to metronidazole; however, infections caused by the majority of these organisms respond to tinidazole or higher doses of metronidazole. Low-level metronidazole resistance has been identified in 2%–5% of cases of vaginal trichomoniasis. High-level resistance is rare. Tinidazole has a longer serum half-life and reaches higher levels in genitourinary tissues than metronidazole. In addition, many T. vaginalis isolates have lower minimum inhibitory concentrations (MICs) to tinidazole than metronidazole.
If treatment failure occurs with metronidazole 2 g single dose and reinfection is excluded, the patient can be treated with metronidazole 500 mg orally twice daily for 7 days or tinidazole 2 g single dose. For patients failing either of these regimens, clinicians should consider treatment with tinidazole or metronidazole at 2 g orally for 5 days. If these therapies are not effective, further management should be discussed with a specialist. The consultation should ideally include determination of the susceptibility of T. vaginalis to metronidazole and tinidazole. Consultation and T. vaginalis susceptibility testing is available from CDC (telephone: 770-488-4115; website: http://www.cdc.gov/std).
Special Considerations:
Allergy, Intolerance, and Adverse Reactions
Metronidazole and tinidazole are both nitroimidazoles. Patients with an immediate-type allergy to a nitroimidazole can be managed by metronidazole desensitization in consultation with a specialist (171,172). Topical therapy with drugs other than nitroimidazoles can be attempted, but cure rates are low (<50%).
Pregnancy
Vaginal trichomoniasis has been associated with adverse pregnancy outcomes, particularly premature rupture of membranes, preterm delivery, and low birthweight. However, data do not suggest that metronidazole treatment results in a reduction in perinatal morbidity. Although some trials suggest the possibility of increased prematurity or low birthweight after metronidazole treatment, limitations of the studies prevent definitive conclusions regarding risks of treatment (173,174). Treatment of T. vaginalis might relieve symptoms of vaginal discharge in pregnant women and might prevent respiratory or genital infection of the newborn and further sexual transmission. Clinicians should counsel patients regarding the potential risks and benefits of treatment. Some specialists would defer therapy in asymptomatic pregnant women until after 37 weeks’ gestation. In addition, these pregnant women should be provided careful counseling regarding condom use and the continued risk of sexual transmission.
Women may be treated with 2 g of metronidazole in a single dose. Metronidazole is pregnancy category B (animal studies have revealed no evidence of harm to the fetus, but no adequate, well-controlled studies among pregnant women have been conducted). Multiple studies and meta-analyses have not demonstrated a consistent association between metronidazole use during pregnancy and teratogenic or mutagenic effects in infants (163–165). Tinidazole is pregnancy category C (animal studies have demonstrated an adverse event, and no adequate, well-controlled studies in pregnant women have been conducted), and its safety in pregnant women has not been well-evaluated.
In lactating women who are administered metronidazole, withholding breastfeeding during treatment and for 12–24 hours after the last dose will reduce the exposure of metronidazole to the infant. While using tinidazole, interruption of breastfeeding is recommended during treatment and for 3 days after the last dose.
HIV Infection
Patients who have trichomoniasis and also are infected with HIV should receive the same treatment regimen as those who are HIV negative. The incidence, persistence, and recurrence of trichomoniasis in HIV-infected women are not correlated with immune status. [16][17]
Primary Prevention
The surest way to avoid transmission of sexually transmitted diseases is to abstain from sexual contact, or to be in a long-term mutually monogamous relationship with a partner who has been tested and is known to be uninfected.
Latex male condoms, when used consistently and correctly, can reduce the risk of transmission of trichomoniasis.
Any genital symptom such as discharge or burning during urination or an unusual sore or rash should be a signal to stop having sex and to consult a health care provider immediately. A person diagnosed with trichomoniasis (or any other STD) should receive treatment and should notify all recent sex partners so that they can see a health care provider and be treated. This reduces the risk that the sex partners will develop complications from trichomoniasis and reduces the risk that the person with trichomoniasis will become re-infected. Sex should be stopped until the person with trichomoniasis and all of his or her recent partners complete treatment for trichomoniasis and have no symptoms. [18]
Acknowledgements
The content on this page was first contributed by: C. Michael Gibson, M.S., M.D.
List of contributors:
Pilar Almonacid
External links
- Trichomoniasis.net
- Vaginitis/Vaginal infection fact sheet from the National Institute of Allergies and Infections. The first version of this article was taken from this public domain resource.
- eMedicine Health Trichomoniasis
Template:STD/STI Template:Protozoal diseases
da:Trichomonas de:Trichomoniasis id:Trichomoniasis nl:Trichomoniasis sr:Трихомонијаза fi:Trikomoniaasi Template:SIB