Phenazopyridine: Difference between revisions
m (Protected "Phenazopyridine": Protecting pages from unwanted edits ([edit=sysop] (indefinite) [move=sysop] (indefinite))) |
No edit summary |
||
| (One intermediate revision by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{ | {{DrugProjectFormSinglePage | ||
|IUPAC_name | |authorTag= | ||
| image = Phenazopyridine | |||
|CAS_number | {{VP}} | ||
<!--Overview--> | |||
|genericName= | |||
Phenazopyridine | |||
|aOrAn= | |||
an | |||
|drugClass= | |||
[[analgesic]] | |||
|indication= | |||
[[dysuria]] | |||
|hasBlackBoxWarning= | |||
|adverseReactions= | |||
[[headache]], [[nausea]], [[vomiting]] and [[diarrhea]] | |||
<!--Black Box Warning--> | |||
|blackBoxWarningTitle= | |||
Title | |||
|blackBoxWarningBody= | |||
<i><span style="color:#FF0000;">ConditionName: </span></i> | |||
* Content | |||
<!--Adult Indications and Dosage--> | |||
<!--FDA-Labeled Indications and Dosage (Adult)--> | |||
|fdaLIADAdult= | |||
=====Dysuria===== | |||
*Phenazopyridine HCl is indicated for the symptomatic relief of pain, burning, [[urgency frequency]], and other discomforts arising from irritation of the [[mucosa]] of the [[lower urinary tract]] caused by [[infection]], [[trauma]], [[surgery]], [[endoscopic procedures]], or the passage of sounds or [[catheters]]. | |||
*The use of phenazopyridine for relief of symptoms should not delay definitive diagnosis and treatment of causative conditions. The drug should be used for symptomatic relief of pain and not as a substitute for specific surgery or antimicrobial therapy. | |||
*200 mg 3 times daily after meals. When used concomitantly with an antibacterial agent for the treatment of a urinary tract infection, the administration of phenazopyridine should not exceed 2 days. If symptoms persist, the patient should be re-evaluated. | |||
<!--Off-Label Use and Dosage (Adult)--> | |||
<!--Guideline-Supported Use (Adult)--> | |||
|offLabelAdultGuideSupport= | |||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
<!--Non–Guideline-Supported Use (Adult)--> | |||
|offLabelAdultNoGuideSupport= | |||
There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
<!--Pediatric Indications and Dosage--> | |||
<!--FDA-Labeled Indications and Dosage (Pediatric)--> | |||
|fdaLIADPed= | |||
There is limited information regarding <i>FDA-Labeled Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Off-Label Use and Dosage (Pediatric)--> | |||
<!--Guideline-Supported Use (Pediatric)--> | |||
|offLabelPedGuideSupport= | |||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Non–Guideline-Supported Use (Pediatric)--> | |||
|offLabelPedNoGuideSupport= | |||
There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Contraindications--> | |||
|contraindications= | |||
*In patients who are hypersensitive to the drug or its ingredients. Phenazopyridine is contraindicated in patients with [[renal insufficiency]], severe [[liver disease]], severe [[hepatitis]] or [[pyelonephritis]] of pregnancy. | |||
*It should be used cautiously in the presence of [[GI disturbances]]. | |||
<!--Warnings--> | |||
|warnings= | |||
*Phenazopyridine hydrochloride is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity in experimental animals (IARC 1980, 1982, 1987, NCI 1978). When administered in the diet, phenazopyridine hydrochloride increased the incidences of [[hepatocellular adenomas]] and [[carcinomas]] in female mice and [[adenomas]] and [[adenocarcinomas of the colon]] and rectum in rats of both sexes. | |||
*There is inadequate evidence for the carcinogenicity of phenazopyridine hydrochloride in humans (IARC 1987). In one limited epidemiological study, no significant excess of any cancer was observed among 2,214 patients who received phenazopyridine hydrochloride and were followed for a minimum of 3 years. | |||
====Precautions==== | |||
*The patient should be advised that phenazopyridine produces an orange to red color in the urine and feces, and may cause staining. Phenazopyridine may cause discoloration of body fluids and staining of [[contact lenses]] has been reported. A yellowish color of the skin or sclera may indicate accumulation of phenazopyridine resulting from impaired [[renal function]] and necessitates discontinuance of the drug. It should be noted that a decline in renal function is common in elderly patients. Phenazopyridine may mask pathological conditions and interfere with laboratory test values using colorimetric, spectrophotometric or fluorometric analysis methods. | |||
*Cautious use in patients with [[G-6-PD deficiency]] is advised since these patients are susceptible to oxidative [[hemolysis]] and may have greater potential to develop [[hemolytic anemia]]. | |||
<!--Adverse Reactions--> | |||
<!--Clinical Trials Experience--> | |||
|clinicalTrials= | |||
*The following adverse events have been reported: | |||
=====CNS===== | |||
[[Headache]]. | |||
=====Gastrointestinal===== | |||
[[Nausea]], [[vomiting]] and [[diarrhea]]. | |||
=====Dermatologic and Hypersensitivity===== | |||
[[Rash]], [[pruritus]], discoloration, [[anaphylactoid]]-like reaction and hypersensitivity [[hepatitis]] | |||
=====Hematologic===== | |||
[[Methemoglobinemia]], [[hemolytic anemia]], potential [[hemolytic]] agent in [[G-6-PD deficiency]], [[sulfhemoglobinemia]]. | |||
=====Other===== | |||
[[Visual disturbances]], renal and hepatic toxicity usually associated with overdose, [[renal calculi]], [[jaundice]], discoloration of body fluids and [[aseptic meningitis]]. | |||
<!--Postmarketing Experience--> | |||
|postmarketing= | |||
There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | |||
<!--Drug Interactions--> | |||
|drugInteractions= | |||
*The interaction of phenazopyridine with other drugs has not been studied in a systematic manner. However, the medical literature to date suggests that no significant interactions have been reported. | |||
<!--Use in Specific Populations--> | |||
|useInPregnancyFDA= | |||
* '''Pregnancy Category B''' | |||
*Reproductive studies with phenazopyridine (in combination with sulfacytine) in rats given up to 110 mg/kg/day and in rabbits given up to 39 mg/kg/day during organogenesis revealed no evidence of harm to offspring. | |||
*One prospective study in humans demonstrated that phenazopyridine traverses the placenta into the fetal compartment. There are no adequate and well-controlled studies in pregnant women. Therefore, phenazopyridine should be used in pregnant women only if the benefit clearly outweighs the risk. | |||
|useInPregnancyAUS= | |||
* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | |||
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | |||
|useInLaborDelivery= | |||
There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | |||
|useInNursing= | |||
*It is not known whether phenazopyridine or its metabolites are excreted in human milk. Because many drugs are excreted in human milk, a decision should be made to discontinue nursing or to discontinue the drug, taking into account the importance of drug therapy to the mother. | |||
|useInPed= | |||
*Adequate and well-controlled studies have not been performed in the pediatric population. No pediatric-specific problems have been documented. | |||
|useInGeri= | |||
There is no FDA guidance on the use of {{PAGENAME}} with respect to geriatric patients. | |||
|useInGender= | |||
There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | |||
|useInRace= | |||
There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | |||
|useInRenalImpair= | |||
There is no FDA guidance on the use of {{PAGENAME}} in patients with renal impairment. | |||
|useInHepaticImpair= | |||
There is no FDA guidance on the use of {{PAGENAME}} in patients with hepatic impairment. | |||
|useInReproPotential= | |||
There is no FDA guidance on the use of {{PAGENAME}} in women of reproductive potentials and males. | |||
|useInImmunocomp= | |||
There is no FDA guidance one the use of {{PAGENAME}} in patients who are immunocompromised. | |||
<!--Administration and Monitoring--> | |||
|administration= | |||
* Oral | |||
|monitoring= | |||
There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | |||
<!--IV Compatibility--> | |||
|IVCompat= | |||
There is limited information regarding <i>IV Compatibility</i> of {{PAGENAME}} in the drug label. | |||
<!--Overdosage--> | |||
|overdose= | |||
===Acute Overdose=== | |||
====Signs and Symptoms==== | |||
*Exceeding the recommended dose in patients with normal renal function or administering the recommended dose to patients with impaired renal function (common in elderly patients) may lead to increased serum levels and toxic reactions. [[Methemoglobinemia]] generally follows a massive, acute overdose. [[Methylene blue]], 1 to 2 mg/kg/dose given i.v. as a 1% solution as needed, should cause prompt reduction of the [[methemoglobinemia]] and disappearance of the [[cyanosis]] which is an aid in diagnosis. Oxidative [[Heinz body]] [[hemolytic anemia]] also may occur, and "[[bite cells]]" ([[degmacytes]]) may be present in a chronic overdosage situation. Red blood cell [[G-6-PD deficiency]] may predispose to [[hemolysis]]; however, [[hemolysis]] may occur at normal doses in patients with [[G-6-PD]] Mediterranean. | |||
*[[Renal toxicity]] and occasional failure and [[hepatic impairment]] may also occur. | |||
====Management==== | |||
*Treatment is symptomatic and supportive. | |||
===Chronic Overdose=== | |||
There is limited information regarding <i>Chronic Overdose</i> of {{PAGENAME}} in the drug label. | |||
<!--Pharmacology--> | |||
<!--Drug box 2--> | |||
|drugBox= | |||
{{Drugbox | |||
| Verifiedfields = changed | |||
| verifiedrevid = 417929125 | |||
| IUPAC_name = 3-phenyldiazenylpyridine-2,6-diamine | |||
| image = Phenazopyridine00.png | |||
<!--Clinical data--> | |||
| tradename = Pyridium | |||
| Drugs.com = {{drugs.com|monograph|phenazopyridine-hydrochloride}} | |||
| MedlinePlus = a682231 | |||
| licence_US = Phenazopyridine | |||
| pregnancy_category = B | |||
| legal_US = OTC | |||
| legal_status = [[Over-the-counter drug|OTC]]<small>([[Canada]])</small> | |||
| routes_of_administration = oral | |||
<!--Identifiers--> | |||
| CASNo_Ref = {{cascite|correct|CAS}} | |||
| CAS_number_Ref = {{cascite|correct|??}} | |||
| CAS_number = 94-78-0 | |||
| ATC_prefix = G04 | | ATC_prefix = G04 | ||
| ATC_suffix = BX06 | | ATC_suffix = BX06 | ||
| PubChem = 4756 | | PubChem = 4756 | ||
| DrugBank= | | DrugBank_Ref = {{drugbankcite|changed|drugbank}} | ||
| | | DrugBank = DB01438 | ||
| | | ChemSpiderID_Ref = {{chemspidercite|changed|chemspider}} | ||
| | | ChemSpiderID = 4592 | ||
| | | UNII_Ref = {{fdacite|changed|FDA}} | ||
| | | UNII = K2J09EMJ52 | ||
| | | KEGG_Ref = {{keggcite|correct|kegg}} | ||
| | | KEGG = D08346 | ||
| | | ChEMBL_Ref = {{ebicite|changed|EBI}} | ||
| | | ChEMBL = 1242 | ||
| | |||
| | <!--Chemical data--> | ||
| C=11 | H=11 | N=5 | |||
| molecular_weight = 213.239 g/mol | |||
}} | }} | ||
<!--Mechanism of Action--> | |||
|mechAction= | |||
* Phenazopyridine hydrochloride is excreted in the urine where it exerts a topical [[analgesic]] effect on the mucosa of the [[urinary tract]]. This action helps to relieve pain, burning, urgency and frequency. The precise mechanism of action is unknown. | |||
<!--Structure--> | |||
|structure= | |||
* Phenazopyridine Hydrochloride is a reddish-brown, odorless, slightly bitter, crystalline powder. It has a specific local analgesic effect in the urinary tract, promptly relieving burning and pain. | |||
*Following is the structural formula: | |||
: [[File:{{PAGENAME}}01.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
*Phenazopyridine HCl oral tablets contain the following inactive ingredients: Carnauba Wax, Croscarmellose Sodium, Hypromellose, Magnesium Stearate, Microcrystalline Cellulose, Polyethylene Glycol, Povidone, Pregelatinized Starch | |||
<!--Pharmacodynamics--> | |||
|PD= | |||
There is limited information regarding <i>Pharmacodynamics</i> of {{PAGENAME}} in the drug label. | |||
<!--Pharmacokinetics--> | |||
|PK= | |||
*The pharmacokinetic properties of phenazopyridine hydrochloride have not been determined. Phenazopyridine and its metabolites are rapidly excreted by the kidneys. In a small number of healthy subjects, 90% of a 600 mg/day oral dose of phenazopyridine hydrochloride was eliminated in the urine in 24 hours, 41% as unchanged drug and 49% as metabolites. | |||
<!--Nonclinical Toxicology--> | |||
|nonClinToxic= | |||
*Long-term administration of phenazopyridine has been associated with tumors of the large intestine in rats and of the liver in mice. Available epidemiological data are insufficient to evaluate the carcinogenicity of phenazopyridine in humans. In vitro studies indicate that phenazopyridine in the presence of metabolic activation is mutagenic in bacteria and mutagenic and clastogenic in mammalian cells. | |||
<!--Clinical Studies--> | |||
= | |clinicalStudies= | ||
There is limited information regarding <i>Clinical Studies</i> of {{PAGENAME}} in the drug label. | |||
<!--How Supplied--> | |||
|howSupplied= | |||
* 100 mg Tablets: Supplied in bottles of 100. Reddish-brown, round tablets, debossed “M450”. NDC# 58657-450-01. | |||
*200 mg Tablets: Supplied in bottles of 100. Reddish-brown, round tablets, debossed “M451”. NDC# 58657-451-01. | |||
*Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C -30°C (59°-86°F). | |||
* | |||
{{ | <!--Patient Counseling Information--> | ||
{{ | |||
|fdaPatientInfo= | |||
*The patient should be advised to take phenazopyridine with or following food or after eating a snack to reduce stomach upset. | |||
*The patients should be aware that phenazopyridine causes a reddish orange discoloration of the urine and feces, and may stain clothing. Phenazopyridine may cause discoloration of body fluids and staining of contact lenses has been reported. There have been reports of teeth discoloration when the product has been broken or held in the mouth prior to swallowing. | |||
*Patients should be instructed to take phenazopyridine for only 2 days if an antibacterial agent is administered concurrently for the treatment of a [[urinary tract infection]]. If symptoms persist beyond those 2 days, the patient should be instructed to contact his or her physician. | |||
<!--Precautions with Alcohol--> | |||
|alcohol= | |||
* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |||
<!--Brand Names--> | |||
|brandNames= | |||
* PHENAZOPYRIDINE HCL®<ref>{{Cite web | title = PHENAZOPYRIDINE HCL - phenazopyridine hydrochloride tablet | url = http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=47185181-607e-4bbe-8850-726af1973cf1 }}</ref> | |||
<!--Look-Alike Drug Names--> | |||
|lookAlike= | |||
<!--Drug Shortage Status--> | |||
|drugShortage= | |||
}} | |||
<!--Pill Image--> | |||
{{PillImage | |||
|fileName=No image.jpg|This image is provided by the National Library of Medicine. | |||
|drugName= | |||
|NDC= | |||
|drugAuthor= | |||
|ingredients= | |||
|pillImprint= | |||
|dosageValue= | |||
|dosageUnit= | |||
|pillColor= | |||
|pillShape= | |||
|pillSize= | |||
|pillScore= | |||
}} | |||
<!--Label Display Image--> | |||
{{LabelImage | |||
|fileName={{PAGENAME}}02.png|This image is provided by the National Library of Medicine. | |||
}} | |||
{{LabelImage | |||
|fileName={{PAGENAME}}03.png|This image is provided by the National Library of Medicine. | |||
}} | |||
<!--Category--> | |||
[[Category:Drug]] | |||
Latest revision as of 15:22, 7 November 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Vignesh Ponnusamy, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Phenazopyridine is an analgesic that is FDA approved for the {{{indicationType}}} of dysuria. Common adverse reactions include headache, nausea, vomiting and diarrhea.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Dysuria
- Phenazopyridine HCl is indicated for the symptomatic relief of pain, burning, urgency frequency, and other discomforts arising from irritation of the mucosa of the lower urinary tract caused by infection, trauma, surgery, endoscopic procedures, or the passage of sounds or catheters.
- The use of phenazopyridine for relief of symptoms should not delay definitive diagnosis and treatment of causative conditions. The drug should be used for symptomatic relief of pain and not as a substitute for specific surgery or antimicrobial therapy.
- 200 mg 3 times daily after meals. When used concomitantly with an antibacterial agent for the treatment of a urinary tract infection, the administration of phenazopyridine should not exceed 2 days. If symptoms persist, the patient should be re-evaluated.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Phenazopyridine in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Phenazopyridine in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Phenazopyridine in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Phenazopyridine in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Phenazopyridine in pediatric patients.
Contraindications
- In patients who are hypersensitive to the drug or its ingredients. Phenazopyridine is contraindicated in patients with renal insufficiency, severe liver disease, severe hepatitis or pyelonephritis of pregnancy.
- It should be used cautiously in the presence of GI disturbances.
Warnings
- Phenazopyridine hydrochloride is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity in experimental animals (IARC 1980, 1982, 1987, NCI 1978). When administered in the diet, phenazopyridine hydrochloride increased the incidences of hepatocellular adenomas and carcinomas in female mice and adenomas and adenocarcinomas of the colon and rectum in rats of both sexes.
- There is inadequate evidence for the carcinogenicity of phenazopyridine hydrochloride in humans (IARC 1987). In one limited epidemiological study, no significant excess of any cancer was observed among 2,214 patients who received phenazopyridine hydrochloride and were followed for a minimum of 3 years.
Precautions
- The patient should be advised that phenazopyridine produces an orange to red color in the urine and feces, and may cause staining. Phenazopyridine may cause discoloration of body fluids and staining of contact lenses has been reported. A yellowish color of the skin or sclera may indicate accumulation of phenazopyridine resulting from impaired renal function and necessitates discontinuance of the drug. It should be noted that a decline in renal function is common in elderly patients. Phenazopyridine may mask pathological conditions and interfere with laboratory test values using colorimetric, spectrophotometric or fluorometric analysis methods.
- Cautious use in patients with G-6-PD deficiency is advised since these patients are susceptible to oxidative hemolysis and may have greater potential to develop hemolytic anemia.
Adverse Reactions
Clinical Trials Experience
- The following adverse events have been reported:
CNS
Gastrointestinal
Nausea, vomiting and diarrhea.
Dermatologic and Hypersensitivity
Rash, pruritus, discoloration, anaphylactoid-like reaction and hypersensitivity hepatitis
Hematologic
Methemoglobinemia, hemolytic anemia, potential hemolytic agent in G-6-PD deficiency, sulfhemoglobinemia.
Other
Visual disturbances, renal and hepatic toxicity usually associated with overdose, renal calculi, jaundice, discoloration of body fluids and aseptic meningitis.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Phenazopyridine in the drug label.
Drug Interactions
- The interaction of phenazopyridine with other drugs has not been studied in a systematic manner. However, the medical literature to date suggests that no significant interactions have been reported.
Use in Specific Populations
Pregnancy
- Pregnancy Category B
- Reproductive studies with phenazopyridine (in combination with sulfacytine) in rats given up to 110 mg/kg/day and in rabbits given up to 39 mg/kg/day during organogenesis revealed no evidence of harm to offspring.
- One prospective study in humans demonstrated that phenazopyridine traverses the placenta into the fetal compartment. There are no adequate and well-controlled studies in pregnant women. Therefore, phenazopyridine should be used in pregnant women only if the benefit clearly outweighs the risk.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Phenazopyridine in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Phenazopyridine during labor and delivery.
Nursing Mothers
- It is not known whether phenazopyridine or its metabolites are excreted in human milk. Because many drugs are excreted in human milk, a decision should be made to discontinue nursing or to discontinue the drug, taking into account the importance of drug therapy to the mother.
Pediatric Use
- Adequate and well-controlled studies have not been performed in the pediatric population. No pediatric-specific problems have been documented.
Geriatic Use
There is no FDA guidance on the use of Phenazopyridine with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Phenazopyridine with respect to specific gender populations.
Race
There is no FDA guidance on the use of Phenazopyridine with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Phenazopyridine in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Phenazopyridine in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Phenazopyridine in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Phenazopyridine in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
There is limited information regarding Monitoring of Phenazopyridine in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Phenazopyridine in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- Exceeding the recommended dose in patients with normal renal function or administering the recommended dose to patients with impaired renal function (common in elderly patients) may lead to increased serum levels and toxic reactions. Methemoglobinemia generally follows a massive, acute overdose. Methylene blue, 1 to 2 mg/kg/dose given i.v. as a 1% solution as needed, should cause prompt reduction of the methemoglobinemia and disappearance of the cyanosis which is an aid in diagnosis. Oxidative Heinz body hemolytic anemia also may occur, and "bite cells" (degmacytes) may be present in a chronic overdosage situation. Red blood cell G-6-PD deficiency may predispose to hemolysis; however, hemolysis may occur at normal doses in patients with G-6-PD Mediterranean.
- Renal toxicity and occasional failure and hepatic impairment may also occur.
Management
- Treatment is symptomatic and supportive.
Chronic Overdose
There is limited information regarding Chronic Overdose of Phenazopyridine in the drug label.
Pharmacology
 | |
| Clinical data | |
|---|---|
| Trade names | Pyridium |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682231 |
| [[Regulation of therapeutic goods |Template:Engvar data]] |
|
| Pregnancy category |
|
| Routes of administration | oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C11H11N5 |
| Molar mass | 213.239 g/mol |
| | |
Mechanism of Action
- Phenazopyridine hydrochloride is excreted in the urine where it exerts a topical analgesic effect on the mucosa of the urinary tract. This action helps to relieve pain, burning, urgency and frequency. The precise mechanism of action is unknown.
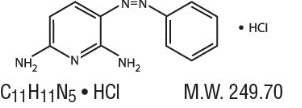
Structure
- Phenazopyridine Hydrochloride is a reddish-brown, odorless, slightly bitter, crystalline powder. It has a specific local analgesic effect in the urinary tract, promptly relieving burning and pain.
- Following is the structural formula:
- Phenazopyridine HCl oral tablets contain the following inactive ingredients: Carnauba Wax, Croscarmellose Sodium, Hypromellose, Magnesium Stearate, Microcrystalline Cellulose, Polyethylene Glycol, Povidone, Pregelatinized Starch
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Phenazopyridine in the drug label.
Pharmacokinetics
- The pharmacokinetic properties of phenazopyridine hydrochloride have not been determined. Phenazopyridine and its metabolites are rapidly excreted by the kidneys. In a small number of healthy subjects, 90% of a 600 mg/day oral dose of phenazopyridine hydrochloride was eliminated in the urine in 24 hours, 41% as unchanged drug and 49% as metabolites.
Nonclinical Toxicology
- Long-term administration of phenazopyridine has been associated with tumors of the large intestine in rats and of the liver in mice. Available epidemiological data are insufficient to evaluate the carcinogenicity of phenazopyridine in humans. In vitro studies indicate that phenazopyridine in the presence of metabolic activation is mutagenic in bacteria and mutagenic and clastogenic in mammalian cells.
Clinical Studies
There is limited information regarding Clinical Studies of Phenazopyridine in the drug label.
How Supplied
- 100 mg Tablets: Supplied in bottles of 100. Reddish-brown, round tablets, debossed “M450”. NDC# 58657-450-01.
- 200 mg Tablets: Supplied in bottles of 100. Reddish-brown, round tablets, debossed “M451”. NDC# 58657-451-01.
- Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C -30°C (59°-86°F).
Storage
There is limited information regarding Phenazopyridine Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Phenazopyridine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Phenazopyridine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- The patient should be advised to take phenazopyridine with or following food or after eating a snack to reduce stomach upset.
- The patients should be aware that phenazopyridine causes a reddish orange discoloration of the urine and feces, and may stain clothing. Phenazopyridine may cause discoloration of body fluids and staining of contact lenses has been reported. There have been reports of teeth discoloration when the product has been broken or held in the mouth prior to swallowing.
- Patients should be instructed to take phenazopyridine for only 2 days if an antibacterial agent is administered concurrently for the treatment of a urinary tract infection. If symptoms persist beyond those 2 days, the patient should be instructed to contact his or her physician.
Precautions with Alcohol
- Alcohol-Phenazopyridine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- PHENAZOPYRIDINE HCL®[1]
Look-Alike Drug Names
There is limited information regarding Phenazopyridine Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=Phenazopyridine |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Phenazopyridine |Label Name=Phenazopyridine02.png
}}
{{#subobject:
|Label Page=Phenazopyridine |Label Name=Phenazopyridine03.png
}}
- Pages with script errors
- Template:drugs.com link with non-standard subpage
- Drugs with non-standard legal status
- Articles with changed DrugBank identifier
- Articles with changed ChemSpider identifier
- Articles with changed EBI identifier
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Articles without InChI source
- Drugboxes which contain changes to verified fields
- Drug