Parathyroid hormone (injection): Difference between revisions
No edit summary |
No edit summary |
||
| Line 145: | Line 145: | ||
* One 100 mcg dose of [[Parathyroid hormone]] ([[Injection]]) provides a 24-hour calcemic response in [[hypoparathyroidism]] subjects. | * One 100 mcg dose of [[Parathyroid hormone]] ([[Injection]]) provides a 24-hour calcemic response in [[hypoparathyroidism]] subjects. | ||
[[File:Parathyroid.png|thumb|center]] | [[File:Parathyroid.png|thumb|center]] | ||
==== Absorption: ==== | |||
* NATPARA administered subcutaneously has an absolute bioavailability of 53%. | |||
==== Distribution: ==== | |||
* NATPARA has a volume of distribution of 5.35 L at steady state. | |||
==== Metabolism: ==== | |||
* In vitro and in vivo studies demonstrated that the clearance of parathyroid hormone is primarily a hepatic process with a lesser role played by the kidneys. | |||
==== Excretion: ==== | |||
* In the liver, most of the intact parathyroid hormone is cleaved by cathepsins. | |||
* In the kidney, a small amount of parathyroid hormone binds to physiologic PTH-1 receptors, but most is filtered at the glomerulus. | |||
* C-terminal fragments are also cleared efficiently by glomerular filtration. | |||
==== Hepatic Impairment ==== | |||
* A pharmacokinetic study was conducted in 6 men and 6 women with moderate hepatic impairment (Child-Pugh Classification of 7-9 [Grade B]) as compared with a matched group of 12 subjects with normal hepatic function. | |||
* Following a single 100-mcg subcutaneous dose, the mean Cmax and baseline-corrected Cmax values were 18% to 20% greater in the moderately impaired subjects than in those with normal function. | |||
* There were no apparent differences in the serum total calcium concentration-time profiles between the 2 hepatic function groups. | |||
* No dose adjustment for NATPARA is recommended in patients with mild to moderate hepatic impairment. | |||
==== Renal Impairment: ==== | |||
* Pharmacokinetics following a single NATPARA 100 mcg subcutaneous dose was evaluated in 16 subjects with normal renal function (creatinine clearance (CLcr) >90 mL/min) and 16 subjects with renal impairment. | |||
* The mean maximum concentration (Cmax) of parathyroid hormone following administration of 100 mcg NATPARA in subjects with mild (CLcr 60 to 90 mL/min) and moderate (CLcr 30 to 60 mL/min) renal impairment was approximately 22% higher than that observed in subjects with normal renal function. | |||
* Exposure to parathyroid hormone as measured by AUC0-last and baseline-corrected AUC0-last was approximately 3.9% and 2.5%, respectively, higher than that observed for subjects with normal renal function. | |||
* No studies were conducted in patients with severe renal impairment or in renal impairment patients on dialysis. | |||
==== Age, Sex, Race, and Weight ==== | |||
* Based on population pharmacokinetic analysis, age, sex, race, and body weight did not significantly affect the NATPARA pharmacokinetics. | |||
|nonClinToxic=====Carcinogenesis, Mutagenesis, Impairment of Fertility ==== | |||
* In a 104-week carcinogenicity study in rats, parathyroid hormone was given subcutaneously at doses of 10, 50 and 150 mcg/kg/day. | |||
* These doses resulted in systemic exposures that were, respectively 3 to 71 times higher than systemic exposure observed in humans following a subcutaneous dose of 100 mcg/day based on AUC. | |||
* Systemic exposure at the 10 mcg/kg/day dose of parathyroid hormone was 3-5 times greater AUC than the exposure observed in hypoparathyroidism subjects at the clinical dose of 100 mcg/day. | |||
* This is the lowest dose at which a parathyroid hormone-related increase in bone tumors was observed in rats. | |||
* Higher exposures resulted in a marked dose-related increase in all bone tumors including osteoma, osteoblastoma and osteosarcomas in both sexes. | |||
* The bone tumors in rats occurred in association with a large increase in bone mass and focal osteoblast hyperplasia. | |||
* However, since bone metabolism in the rat differs from that in humans, the relevance of these animal findings to humans is uncertain. | |||
* Parathyroid hormone is not genotoxic in any of the following test systems: the bacterial reverse mutation (Ames) assay or the in vitro mammalian cell forward-gene mutation (AS52/XPRT) assay study with and without metabolic activation. | |||
* No effect on fertility was observed in male and female rats given parathyroid hormone at doses up to 1000 mcg/kg/day (120 times systemic exposure after a clinical dose of 100 mcg/day). | |||
==== Animal Toxicology and/or Pharmacology ==== | |||
* In pregnant rats given subcutaneous doses up to 1000 mcg/kg/day during organogenesis there were no findings observed at 123 times the 100 mcg/day clinical dose based on AUC. | |||
* In pregnant rabbits given subcutaneous doses of 5, 10 and 50 mcg/kg/day during organogenesis, various skeletal alterations including in complete ossification in <35% of litters given 10 mcg/kg/day which were statistically significant but within historical control range at exposures 8 times the 100 mcg/kg/day clinical dose. | |||
* There was a fetus with spina bifida in the 50 mcg/kg/day dose group at 72 times the 100 mcg/day clinical dose based on AUC. | |||
* Given the association of folic acid deficiency and neural tube defects this finding may be related to decreased body weight and food consumption in the pregnant rabbits. | |||
* Developmental effects were observed in a peri-/post-natal study in pregnant rats given subcutaneous doses of 100, 300, 1000 mcg/kg/day from organogenesis through lactation while an entire litter was stillborn in the 300 mcg/kg/day group (34 times the 100 mcg/day clinical dose based on AUC) and an entire litter from the 1000 mcg/kg/day (123 times the 100 mcg/day clinical dose based on AUC) was dead by postnatal day 4. | |||
* Increased incidence of morbidity associated with dehydration, broken palate and palate injuries related to incisor misalignment and mortality were found in pups from litters given 100 mcg/kg/day (10 times the 100 mcg/day clinical dose based on AUC). At 300 mcg/kg/day there was a litter with kidney dilatation and another with an extra liver lobe. | |||
* There was a single pup with a diaphragmatic hernia from a litter exposed to 1000 mcg/kg/day. | |||
|clinicalStudies===== Study in Patients with Established Hypoparathyroidism ==== | |||
* The efficacy of NATPARA was evaluated in a 24-week, randomized, double-blind, placebo-controlled, multicenter trial. | |||
* In this trial, patients with established hypoparathyroidism receiving calcium and active forms of vitamin D (vitamin D metabolite or analogs) were randomized (2:1) to NATPARA (n=84) or placebo (n=40). | |||
* The mean age was 47 years (range, 19 to 74 years), 79.0% were females and 96.0% were Caucasian, 0.8% were Black, and 1.6% were Asian. | |||
* Patients had hypoparathyroidism for on average 15 years and hypoparathyroidism was caused by post-surgical complications in 71% of cases, idiopathic hypoparathyroidism in 25%, DiGeorge Syndrome in 3%, and auto-immune hypoparathyroidism in 1%. | |||
* Patients with hypoparathyroidism due to calcium-sensing receptor mutations were excluded from the trial. | |||
* The mean eGFR at baseline was 97.4 mL/min/1.73 m2 and 45%, 10% and 0% had mild, moderate and severe renal impairment, respectively, at baseline. | |||
* Before randomization, participants entered a 2-16 weeks run-in phase. | |||
* In this phase calcium supplement and active vitamin D doses were adjusted to target an albumin-corrected serum calcium concentration between 8.0 and 9.0 mg/dL and 25-hydroxyvitamin D was replaced in patients with insufficient stores. | |||
* At randomization, baseline serum calcium was 8.6 mg and participants were receiving a median (interquartile range) daily oral calcium dose of 2000 (1250, 3000) mg and a median daily oral active vitamin D dose equivalent to 0.75 mcg (0.5, 1) of calcitriol. | |||
* At randomization, active forms of vitamin D were reduced by 50% and patients were randomized to NATPARA 50 mcg daily or placebo. | |||
* Randomization was followed by a 12-week NATPARA titration phase and a 12-week NATPARA dose maintenance phase. | |||
* During the titration phase NATPARA was increased by 25 mcg increments every four weeks up to a maximum of 100 mcg. | |||
* Titration was indicated for patients who could not achieve independence from active vitamin D and who could not reduce oral calcium to 500 mg or less per day. | |||
* At end of treatment, 56% of subjects randomized to NATPARA were receiving 100 mcg of NATPARA per day, 26% were receiving 75 mcg of NATPARA per day, and 18% were receiving 50 mcg of NATPARA per day. | |||
* Doses of co-administered active forms of vitamin D and calcium were adjusted (reduced or increased) to maintain albumin-corrected serum calcium within a desired target range throughout the trial in both arms. | |||
* For the efficacy analysis, subjects that fulfilled three components of a three-part response criterion were considered responders. | |||
* A responder was defined as an individual who had: at least a 50% reduction from baseline in the dose of active vitamin D, at least a 50% reduction from baseline in the dose of oral calcium supplementation and an albumin-corrected total serum calcium concentration between 7.5 mg/dL and 10.6 mg/dL. | |||
* At the end of treatment, significantly (p-value <0.001) more subjects treated with NATPARA [46/84 (54.8%)] compared to placebo [1/40 (2.5%)] met the response criterion. | |||
* Forty-two percent (35/84) of subjects randomized to NATPARA were independent of active forms of vitamin D and were on no more than 500 mg of oral calcium, compared with 2.5% (1/40) of subjects randomized to placebo (p<0.001). | |||
* There were no differences in the proportion of patients with a calcium level between 7.5 mg and 10.6 mg at end of treatment between subjects randomized to NATPARA and placebo. | |||
* Table 4 shows the proportion of individuals who, at the end of treatment, fulfilled the 3-part response criterion. | |||
* Table 5 provides results on individual components of the response criterion. | |||
|alcohol=Alcohol-Parathyroid hormone (Injection) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=Alcohol-Parathyroid hormone (Injection) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
}} | }} | ||
Revision as of 19:14, 27 February 2017
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Shivani Chaparala M.B.B.S [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING
See full prescribing information for complete Boxed Warning.
POTENTIAL RISK OF OSTEOSARCOMA:
|
Overview
Parathyroid hormone (injection) is a parathyroid hormone that is FDA approved for the treatment of hypocalcemia in patients with hypoparathyroidism, as an adjunct to calcium and vitamin D.. There is a Black Box Warning for this drug as shown here. Common adverse reactions include Paresthesia, Hypocalcemia, Headache, Hypercalcemia, Nausea, Hypoaesthesia, Diarrhea, Vomiting, Arthralgia, Hypercalciuria and Pain in extremity..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
INDICATIONS AND USAGE
Parathyroid hormone (Injection) is a parathyroid hormone indicated as an adjunct to calcium and vitamin D to control hypocalcemia in patients with hypoparathyroidism.
Limitations of Use
- Because of the potential risk of osteosarcoma, Parathyroid hormone (Injection) is recommended only for patients who cannot be well-controlled on calcium supplements and active forms of vitamin D alone.
- Parathyroid hormone (Injection) was not studied in patients with hypoparathyroidism caused by calcium-sensing receptor mutations.
- Parathyroid hormone (Injection) was not studied in patients with acute post-surgical hypoparathyroidism.
DOSAGE AND ADMINISTRATION
- The dose of Parathyroid hormone (Injection) should be individualized to achieve a serum calcium level in the lower half of the normal range.
- Confirm vitamin D stores are sufficient and serum calcium is above 7.5 mg/dL before starting Parathyroid hormone (Injection).
- The starting dose of Parathyroid hormone (Injection) is 50 mcg injected once daily in the thigh. When starting Parathyroid hormone (Injection), decrease dose of active vitamin D by 50%, if serum calcium is above 7.5 mg/dL.
- Monitor serum calcium levels every 3 to 7 days after starting or adjusting Parathyroid hormone (Injection) dose and when adjusting either active vitamin D or calcium supplements dose while using Parathyroid hormone (Injection).
DOSAGE FORMS AND STRENGTHS
- Parathyroid hormone (Injection) is supplied as a multiple-dose, dual-chamber glass cartridge containing a sterile lyophilized powder and a sterile diluent for reconstitution in four dosage strengths.
- For injection: 25 mcg, 50 mcg, 75 mcg, or 100 mcg.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Parathyroid hormone (Injection) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Parathyroid hormone (Injection) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Parathyroid hormone (injection) FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Parathyroid hormone (Injection) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Parathyroid hormone (Injection) in pediatric patients.
Contraindications
There is limited information regarding Parathyroid hormone (injection) Contraindications in the drug label.
Warnings
|
WARNING
See full prescribing information for complete Boxed Warning.
POTENTIAL RISK OF OSTEOSARCOMA:
|
Potential Risk of Osteosarcoma
- In male and female rats, parathyroid hormone caused an increase in the incidence of osteosarcoma (a malignant bone tumor).
- The occurrence of osteosarcoma was observed to be dependent on parathyroid hormone dose and treatment duration.
- This effect was observed at parathyroid hormone exposure levels ranging from 3 to 71 times the exposure levels for humans receiving a 100 mcg dose of Parathyroid hormone (Injection).
- These data could not exclude a risk to humans.
- Because of a potential risk of osteosarcoma, use Parathyroid hormone (Injection) only in patients who cannot be well-controlled on calcium supplements and active forms of vitamin D alone and for whom the potential benefits are considered to outweigh this potential risk.
- To further mitigate the potential risk of osteosarcoma, avoid use of Parathyroid hormone (Injection) in patients who are at increased risk for osteosarcoma, such as patients with Paget's disease of bone or unexplained elevations of alkaline phosphatase, pediatric and young adult patients with open epiphyses, patients with hereditary disorders predisposing to osteosarcoma, or patients with a prior history of external beam or implant radiation therapy involving the skeleton.
- Instruct patients to promptly report clinical symptoms (e.g., persistent localized pain) and signs (e.g., soft tissue mass tender to palpation) that could be consistent with osteosarcoma.
- Parathyroid hormone (Injection) is available only through a restricted program under a REMS.
Parathyroid hormone (Injection) REMS Program
- Because of the potential risk of osteosarcoma associated with Parathyroid hormone (Injection) therapy, Parathyroid hormone (Injection) is available only through a restricted REMS program called the NATPARA REMS Program.
- Under the Parathyroid hormone (Injection) REMS Program, only certified healthcare providers can prescribe and only certified pharmacies can dispense Parathyroid hormone (Injection).
Hypercalcemia
- Severe hypercalcemia has been reported with Parathyroid hormone (Injection).
- In the pivotal trial, 3 patients randomized to Parathyroid hormone (Injection) required administration of IV fluids to correct hypercalcemia during treatment with Parathyroid hormone (Injection).
- The risk is highest when starting or increasing the dose of Parathyroid hormone (Injection), but can occur at any time.
- Monitor serum calcium and patients for signs and symptoms of hypercalcemia.
- Treat hypercalcemia per standard practice and consider holding and/or lowering the dose of Parathyroid hormone (Injection) if severe hypercalcemia occurs.
Hypocalcemia
- Severe hypocalcemia has been reported with Parathyroid hormone (Injection).
- The risk is highest when Parathyroid hormone (Injection) is withheld, missed or abruptly discontinued, but can occur at any time.
- Monitor serum calcium and patients for signs and symptoms of hypocalcemia.
- Resume treatment with, or increase the dose of, an active form of vitamin D or calcium supplements or both if indicated in patients interrupting or discontinuing Parathyroid hormone (Injection) to prevent severe hypocalcemia.
Risk of Digoxin Toxicity with Concomitant Use of Digitalis Compounds
- The inotropic effects of digoxin are affected by serum calcium levels.
- Hypercalcemia of any cause may predispose to digoxin toxicity.
- In patients using Parathyroid hormone (Injection) concomitantly with digitalis compounds, monitor serum calcium and digoxin levels and patients for signs and symptoms of digitalis toxicity.
- Adjustment of digoxin and/or Parathyroid hormone (Injection) may be needed.
- No drug-drug interaction study has been conducted with digoxin and Parathyroid hormone (Injection).
Adverse Reactions
Clinical Trials Experience
Adverse Reactions in Clinical Trials for Hypoparathyroidism
- Because clinical trials are conducted under widely varying conditions, the adverse reaction rates observed cannot be directly compared to rates in other clinical trials and may not reflect the rates observed in clinical practice.
- Parathyroid hormone (Injection) was studied in a placebo-controlled trial.
- The data described in Table 1 below reflect exposure to Parathyroid hormone (Injection) in 84 patients, including 78 exposed for 24 weeks.
- The mean age of the trial population was 47 years and ranged from 19 to 74 years old.
- Seventy-nine percent (79%) were females.
- Ninety-six percent (96%) were Caucasian, 0.8% were Black, and 1.6% were Asian.
- Patients had had hypoparathyroidism for on average 15 years and hypoparathyroidism was caused by post-surgical complications in 71% of cases, idiopathic hypoparathyroidism in 25% of cases, DiGeorge Syndrome in 3% of cases, and auto-immune hypoparathyroidism in 1% of cases.
- Prior to trial enrollment, participants were receiving a median (interquartile range) daily oral calcium dose of 2000 (1250, 3000) mg and a median daily oral active vitamin D dose equivalent to 0.75 (0.5, 1) mcg of calcitriol.
- The mean eGFR at baseline was 97.4 mL/min/1.73 m2 and 45%, 10% and 0% had mild, moderate and severe renal impairment, respectively, at baseline.
- During the trial, most patients received 100 mcg and the dose range was 50 to 100 mcg administered subcutaneously once daily in the thigh.
- Table 1 lists common adverse reactions associated with Parathyroid hormone (Injection) use in the clinical trial.
- Common adverse reactions were reactions that occurred in ≥5% of subjects and occurred more commonly on Parathyroid hormone (Injection) than on placebo.
TABLE 1:
Hypercalcemia
- In the overall pivotal trial a greater proportion of patients on Parathyroid hormone (Injection) had albumin-corrected serum calcium above the normal range (8.4 to 10.6 mg/dL).
- During the entire trial duration 3 patients on Parathyroid hormone (Injection) and 1 patient on placebo had a calcium level above 12 mg/dL.
- Table 2 displays the number of subjects who had albumin-corrected serum calcium levels above the normal range (8.4 to 10.6 mg/dL) by study treatment period in the placebo-controlled study based on routine monitoring at each trial visit.
- More patients randomized to Parathyroid hormone (Injection) had hypercalcemia in both phases of the study (note: all trial participants underwent a 50% reduction in active vitamin D dose at randomization).
Table 2
Hypocalcemia
- Table 3 displays the number of subjects who had albumin-corrected serum calcium levels below 8.4 mg/dL by treatment period in the placebo-controlled study based on routine monitoring at each trial visit.
- More patients randomized to placebo had hypocalcemia of less than 7 mg/dL in the titration phase (note: all trial participants underwent a 50% reduction in active vitamin D dose at randomization).
- More patients randomized to Parathyroid hormone (Injection) had hypocalcemia of less than 7 mg/dL in the dose maintenance phase.
Table 3:
- The risk of hypocalcemia increases when Parathyroid hormone (Injection) is withdrawn.
- At the end of the trial, Parathyroid hormone (Injection) and placebo were withdrawn, calcium and active vitamin D were returned to baseline doses and subjects were followed for 4 weeks.
- During this withdrawal phase, more patients previously randomized to Parathyroid hormone (Injection) experienced an albumin-corrected serum calcium value of less than 7 mg/dL (5.0% versus 17% for previous treatment with placebo and Parathyroid hormone (Injection) respectively).
- Twenty subjects (24%) previously randomized to Parathyroid hormone (Injection) experienced adverse reactions of hypocalcemia in the post-treatment phase compared to three subjects (8%) previously randomized to placebo.
- Five subjects previously randomized to Parathyroid hormone (Injection) with albumin-corrected serum calcium below 7 mg/dL required treatment with IV calcium gluconate to correct hypocalcemia.
Hypercalciuria
- Treatment with Parathyroid hormone (Injection) did not lower 24-hour urinary calcium excretion in the placebo-controlled trial.
- The proportion of subjects with hypercalciuria (defined as urine calcium levels of >300 mg/24 hours) was similar at baseline and trial end in the Parathyroid hormone (Injection) and placebo groups.
- The median (IQR) 24-hour Urine Calcium at trial end was similar between NATPARA [231 (168-351) mg/24 hours], and placebo [232 (139-342) mg/24 hours].
- At trial end, serum calcium values between Parathyroid hormone (Injection) and placebo were also similar.
- Risk of hypercalciuria throughout the trial was related to serum calcium levels.
- To minimize the risk of hypercalciuria, Parathyroid hormone (Injection) should be dosed to a target albumin-corrected total serum calcium within the lower half of the normal range (i.e., between 8 and 9 mg/dL).
Immunogenicity
- Parathyroid hormone (Injection) may trigger the development of antibodies.
- In the placebo-controlled study in adults with hypoparathyroidism, the incidence of anti-PTH antibodies was 8.6% (3/35) and 5.9% (1/17) in subjects who received subcutaneous administration of 50 to 100 mcg Parathyroid hormone (Injection) or placebo once daily for 24 weeks, respectively.
- Across all clinical studies in subjects with hypoparathyroidism following treatment with Parathyroid hormone (Injection) for up to 2.6 years, the immunogenicity incidence rate was 16.1% (14/87).
- These 14 subjects had low titer anti-PTH antibodies and, of these, 3 subjects subsequently became antibody negative.
- One of these subjects had antibodies with neutralizing activity; this subject maintained a clinical response with no evidence of immune-related adverse reactions.
- Anti-PTH antibodies did not appear to affect efficacy or safety during the clinical trials but their longer-term impact is unknown.
- Immunogenicity assay results are highly dependent on the sensitivity and specificity of the assay and may be influenced by several factors such as: assay methodology, sample handling, timing of sample collection, concomitant medication, and underlying diseases.
- For these reasons, comparison of the incidence of antibodies to Parathyroid hormone (Injection) with the incidence of antibodies to other products may be misleading.
Postmarketing Experience
There is limited information regarding Parathyroid hormone (injection) Postmarketing Experience in the drug label.
Drug Interactions
Alendronate
- Co-administration of alendronate and Parathyroid hormone (Injection) leads to reduction in the calcium-sparing effect, which can interfere with the normalization of serum calcium.
- Concomitant use of Parathyroid hormone (Injection) with alendronate is not recommended.
Digoxin
- Parathyroid hormone (Injection) causes transient increase in calcium and therefore, concomitant use of Parathyroid hormone (Injection) and cardiac glycosides (e.g., digoxin) may predispose patients to digitalis toxicity if hypercalcemia develops.
- Digoxin efficacy is reduced if hypocalcemia is present.
- In patients using Parathyroid hormone (Injection) concomitantly with digoxin, carefully monitor serum calcium and digoxin levels, and patients for signs and symptoms of digoxin toxicity.
- Adjustment of digoxin and/or Parathyroid hormone (Injection) may be needed.
- No drug-drug interaction study has been conducted with digoxin and Parathyroid hormone (Injection).
Use in Specific Populations
Pregnancy
- Developmental effects were observed in a peri-/post-natal study in pregnant rats given subcutaneous doses of 100, 300, 1000 mcg/kg/day from organogenesis through lactation, while entire stillborn litters were observed in the 300 mcg/kg/day group (34 times the 100 mcg/day clinical dose based on AUC).
- Increased incidence of morbidity associated with dehydration, broken palate and palate injuries related to incisor misalignment and mortality were found in pups from litters given 100 mcg/kg/day (10 times the 100 mcg/day clinical dose based on AUC).
- Because animal reproduction studies are not always predictive of human response, Parathyroid hormone (Injection) should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Parathyroid hormone (injection) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Parathyroid hormone (injection) during labor and delivery.
Nursing Mothers
- It is unknown whether Parathyroid hormone (Injection) is excreted in human milk. * In rats, mean parathyroid hormone concentration in milk was approximately 10 ng/mL at a dose of 1000 mcg/kg/day, 42 times lower in milk than in plasma.
- For nursing mothers, consideration should be made whether discontinuing nursing or Parathyroid hormone (Injection) is warranted, taking into account the importance of the drug to the mother.
Pediatric Use
- Safety and efficacy in patients less than 18 years of age has not been established.
- Avoid use of Parathyroid hormone (Injection) in patients who are at increased baseline risk for osteosarcoma including pediatric and young adult patients with open epiphyses.
Geriatic Use
- Clinical studies of Parathyroid hormone (Injection) did not include sufficient numbers of subjects aged 65 and over to determine whether response in these subjects is different from younger subjects.
- In general, dose selection for elderly individuals should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Gender
There is no FDA guidance on the use of Parathyroid hormone (injection) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Parathyroid hormone (injection) with respect to specific racial populations.
Renal Impairment
- Clinical studies of Parathyroid hormone (Injection) did not include sufficient numbers of subjects with moderate and severe renal impairment to determine whether they respond differently from subjects with mild renal impairment or normal renal function.
- Some of the mechanisms of action of Parathyroid hormone (Injection) (e.g., conversion of 25-OH vitamin D to 1,25-OH2 vitamin D) are dependent on renal function.
- Parathyroid hormone (Injection) is eliminated by the kidney and maximum drug levels increased with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Parathyroid hormone (injection) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Parathyroid hormone (injection) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Parathyroid hormone (injection) in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Parathyroid hormone (injection) Administration in the drug label.
Monitoring
There is limited information regarding Parathyroid hormone (injection) Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Parathyroid hormone (injection) and IV administrations.
Overdosage
- Accidental overdose in studies in hypoparathyroidism occurred in 1 subject who received a 150 mcg dose and experienced mild palpitations.
- Serum calcium 24 hours later was 10.3 mg/dL.
- In the event of overdose, the patient should be carefully monitored for hypercalcemia by a medical professional.
Pharmacology
There is limited information regarding Parathyroid hormone (injection) Pharmacology in the drug label.
Mechanism of Action
- Parathyroid hormone (Injection) is a parathyroid hormone.
- Parathyroid hormone raises serum calcium by increasing renal tubular calcium reabsorption, increasing intestinal calcium absorption (i.e., by converting 25-OH vitamin D to 1,25-OH2 vitamin D) and by increasing bone turnover which releases calcium into the circulation.
Structure
There is limited information regarding Parathyroid hormone (injection) Structure in the drug label.
Pharmacodynamics
- The pharmacodynamics in subjects with hypoparathyroidism after single subcutaneous administration of 50 and 100 mcg dose of Parathyroid hormone (Injection) in the thigh were evaluated.
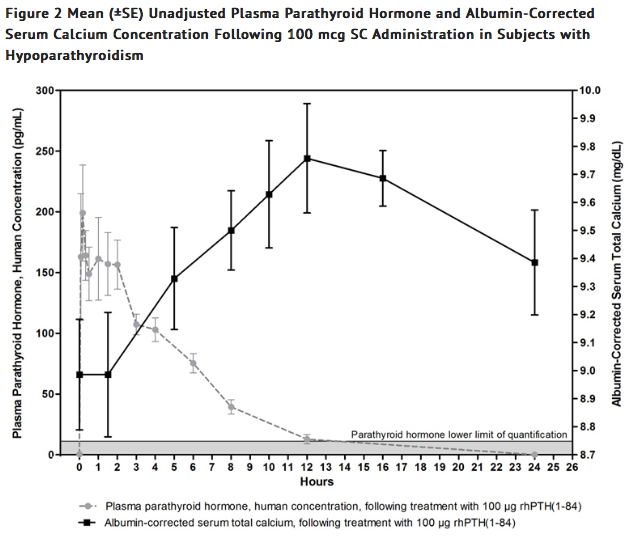
- Treatment with Parathyroid hormone (Injection) increases serum calcium levels (Figure 2).
- The increase in serum calcium levels in hypoparathyroidism subjects occurs in a dose-related manner.
- Mean peak serum calcium levels are reached between 10 and 12 hours following a single subcutaneous injection and the increase in serum calcium above baseline is sustained for more than 24 hours after administration.
- The maximum mean increases of serum calcium, which occurred at 12 hours, were approximately 0.5 mg/dL and 0.7 mg/dL from baseline with the 50 mcg and 100 mcg doses, respectively.
- The mean calcium intake for the 50 and 100 mcg doses was 1700 mg.
Pharmacokinetics
- Following single subcutaneous injections of Parathyroid hormone (Injection) at 50 mcg and 100 mcg in subjects with hypoparathyroidism, peak plasma concentrations (mean Tmax) of Parathyroid hormone (Injection) occurs within 5 to 30 minutes and a second usually smaller peak at 1 to 2 hours.
- The plasma AUC increased in a dose-proportional manner from 50 mcg to 100 mcg.
- The apparent terminal half-life (t1/2) was 3.02 and 2.83 hours for the 50 and 100 mcg dose, respectively.
- Mean unadjusted concentration-time profiles of parathyroid hormone in plasma following SC administration of 100 mcg of Parathyroid hormone (Injection) are presented in Figure 2.
- One 100 mcg dose of Parathyroid hormone (Injection) provides a 24-hour calcemic response in hypoparathyroidism subjects.
Absorption:
- NATPARA administered subcutaneously has an absolute bioavailability of 53%.
Distribution:
- NATPARA has a volume of distribution of 5.35 L at steady state.
Metabolism:
- In vitro and in vivo studies demonstrated that the clearance of parathyroid hormone is primarily a hepatic process with a lesser role played by the kidneys.
Excretion:
- In the liver, most of the intact parathyroid hormone is cleaved by cathepsins.
- In the kidney, a small amount of parathyroid hormone binds to physiologic PTH-1 receptors, but most is filtered at the glomerulus.
- C-terminal fragments are also cleared efficiently by glomerular filtration.
Hepatic Impairment
- A pharmacokinetic study was conducted in 6 men and 6 women with moderate hepatic impairment (Child-Pugh Classification of 7-9 [Grade B]) as compared with a matched group of 12 subjects with normal hepatic function.
- Following a single 100-mcg subcutaneous dose, the mean Cmax and baseline-corrected Cmax values were 18% to 20% greater in the moderately impaired subjects than in those with normal function.
- There were no apparent differences in the serum total calcium concentration-time profiles between the 2 hepatic function groups.
- No dose adjustment for NATPARA is recommended in patients with mild to moderate hepatic impairment.
Renal Impairment:
- Pharmacokinetics following a single NATPARA 100 mcg subcutaneous dose was evaluated in 16 subjects with normal renal function (creatinine clearance (CLcr) >90 mL/min) and 16 subjects with renal impairment.
- The mean maximum concentration (Cmax) of parathyroid hormone following administration of 100 mcg NATPARA in subjects with mild (CLcr 60 to 90 mL/min) and moderate (CLcr 30 to 60 mL/min) renal impairment was approximately 22% higher than that observed in subjects with normal renal function.
- Exposure to parathyroid hormone as measured by AUC0-last and baseline-corrected AUC0-last was approximately 3.9% and 2.5%, respectively, higher than that observed for subjects with normal renal function.
- No studies were conducted in patients with severe renal impairment or in renal impairment patients on dialysis.
Age, Sex, Race, and Weight
- Based on population pharmacokinetic analysis, age, sex, race, and body weight did not significantly affect the NATPARA pharmacokinetics.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- In a 104-week carcinogenicity study in rats, parathyroid hormone was given subcutaneously at doses of 10, 50 and 150 mcg/kg/day.
- These doses resulted in systemic exposures that were, respectively 3 to 71 times higher than systemic exposure observed in humans following a subcutaneous dose of 100 mcg/day based on AUC.
- Systemic exposure at the 10 mcg/kg/day dose of parathyroid hormone was 3-5 times greater AUC than the exposure observed in hypoparathyroidism subjects at the clinical dose of 100 mcg/day.
- This is the lowest dose at which a parathyroid hormone-related increase in bone tumors was observed in rats.
- Higher exposures resulted in a marked dose-related increase in all bone tumors including osteoma, osteoblastoma and osteosarcomas in both sexes.
- The bone tumors in rats occurred in association with a large increase in bone mass and focal osteoblast hyperplasia.
- However, since bone metabolism in the rat differs from that in humans, the relevance of these animal findings to humans is uncertain.
- Parathyroid hormone is not genotoxic in any of the following test systems: the bacterial reverse mutation (Ames) assay or the in vitro mammalian cell forward-gene mutation (AS52/XPRT) assay study with and without metabolic activation.
- No effect on fertility was observed in male and female rats given parathyroid hormone at doses up to 1000 mcg/kg/day (120 times systemic exposure after a clinical dose of 100 mcg/day).
Animal Toxicology and/or Pharmacology
- In pregnant rats given subcutaneous doses up to 1000 mcg/kg/day during organogenesis there were no findings observed at 123 times the 100 mcg/day clinical dose based on AUC.
- In pregnant rabbits given subcutaneous doses of 5, 10 and 50 mcg/kg/day during organogenesis, various skeletal alterations including in complete ossification in <35% of litters given 10 mcg/kg/day which were statistically significant but within historical control range at exposures 8 times the 100 mcg/kg/day clinical dose.
- There was a fetus with spina bifida in the 50 mcg/kg/day dose group at 72 times the 100 mcg/day clinical dose based on AUC.
- Given the association of folic acid deficiency and neural tube defects this finding may be related to decreased body weight and food consumption in the pregnant rabbits.
- Developmental effects were observed in a peri-/post-natal study in pregnant rats given subcutaneous doses of 100, 300, 1000 mcg/kg/day from organogenesis through lactation while an entire litter was stillborn in the 300 mcg/kg/day group (34 times the 100 mcg/day clinical dose based on AUC) and an entire litter from the 1000 mcg/kg/day (123 times the 100 mcg/day clinical dose based on AUC) was dead by postnatal day 4.
- Increased incidence of morbidity associated with dehydration, broken palate and palate injuries related to incisor misalignment and mortality were found in pups from litters given 100 mcg/kg/day (10 times the 100 mcg/day clinical dose based on AUC). At 300 mcg/kg/day there was a litter with kidney dilatation and another with an extra liver lobe.
- There was a single pup with a diaphragmatic hernia from a litter exposed to 1000 mcg/kg/day.
Clinical Studies
Study in Patients with Established Hypoparathyroidism
- The efficacy of NATPARA was evaluated in a 24-week, randomized, double-blind, placebo-controlled, multicenter trial.
- In this trial, patients with established hypoparathyroidism receiving calcium and active forms of vitamin D (vitamin D metabolite or analogs) were randomized (2:1) to NATPARA (n=84) or placebo (n=40).
- The mean age was 47 years (range, 19 to 74 years), 79.0% were females and 96.0% were Caucasian, 0.8% were Black, and 1.6% were Asian.
- Patients had hypoparathyroidism for on average 15 years and hypoparathyroidism was caused by post-surgical complications in 71% of cases, idiopathic hypoparathyroidism in 25%, DiGeorge Syndrome in 3%, and auto-immune hypoparathyroidism in 1%.
- Patients with hypoparathyroidism due to calcium-sensing receptor mutations were excluded from the trial.
- The mean eGFR at baseline was 97.4 mL/min/1.73 m2 and 45%, 10% and 0% had mild, moderate and severe renal impairment, respectively, at baseline.
- Before randomization, participants entered a 2-16 weeks run-in phase.
- In this phase calcium supplement and active vitamin D doses were adjusted to target an albumin-corrected serum calcium concentration between 8.0 and 9.0 mg/dL and 25-hydroxyvitamin D was replaced in patients with insufficient stores.
- At randomization, baseline serum calcium was 8.6 mg and participants were receiving a median (interquartile range) daily oral calcium dose of 2000 (1250, 3000) mg and a median daily oral active vitamin D dose equivalent to 0.75 mcg (0.5, 1) of calcitriol.
- At randomization, active forms of vitamin D were reduced by 50% and patients were randomized to NATPARA 50 mcg daily or placebo.
- Randomization was followed by a 12-week NATPARA titration phase and a 12-week NATPARA dose maintenance phase.
- During the titration phase NATPARA was increased by 25 mcg increments every four weeks up to a maximum of 100 mcg.
- Titration was indicated for patients who could not achieve independence from active vitamin D and who could not reduce oral calcium to 500 mg or less per day.
- At end of treatment, 56% of subjects randomized to NATPARA were receiving 100 mcg of NATPARA per day, 26% were receiving 75 mcg of NATPARA per day, and 18% were receiving 50 mcg of NATPARA per day.
- Doses of co-administered active forms of vitamin D and calcium were adjusted (reduced or increased) to maintain albumin-corrected serum calcium within a desired target range throughout the trial in both arms.
- For the efficacy analysis, subjects that fulfilled three components of a three-part response criterion were considered responders.
- A responder was defined as an individual who had: at least a 50% reduction from baseline in the dose of active vitamin D, at least a 50% reduction from baseline in the dose of oral calcium supplementation and an albumin-corrected total serum calcium concentration between 7.5 mg/dL and 10.6 mg/dL.
- At the end of treatment, significantly (p-value <0.001) more subjects treated with NATPARA [46/84 (54.8%)] compared to placebo [1/40 (2.5%)] met the response criterion.
- Forty-two percent (35/84) of subjects randomized to NATPARA were independent of active forms of vitamin D and were on no more than 500 mg of oral calcium, compared with 2.5% (1/40) of subjects randomized to placebo (p<0.001).
- There were no differences in the proportion of patients with a calcium level between 7.5 mg and 10.6 mg at end of treatment between subjects randomized to NATPARA and placebo.
- Table 4 shows the proportion of individuals who, at the end of treatment, fulfilled the 3-part response criterion.
- Table 5 provides results on individual components of the response criterion.
How Supplied
There is limited information regarding Parathyroid hormone (injection) How Supplied in the drug label.
Storage
There is limited information regarding Parathyroid hormone (injection) Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Parathyroid hormone (injection) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Parathyroid hormone (injection) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Parathyroid hormone (injection) Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Parathyroid hormone (Injection) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Parathyroid hormone (injection) Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Parathyroid hormone (injection) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.