PCSK9

|
WikiDoc Resources for PCSK9 |
|
Articles |
|---|
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on PCSK9 at Clinical Trials.gov Clinical Trials on PCSK9 at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on PCSK9
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Directions to Hospitals Treating PCSK9 Risk calculators and risk factors for PCSK9
|
|
Healthcare Provider Resources |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Proprotein convertase subtilisin/kexin type 9, also known as PCSK9, is a serine protease encoded by the PCSK9 gene. PCSK9 has a medical significance because it plays an important role in lipid homeostasis by promoting degradation of the LDL receptors responsible for clearing circulating LDL-cholesterol (LDL-C) from the plasma. Therefore, drugs that inhibit the actions of PCSK9 can theoretically lower the circulating cholesterol level, and thus lower the risk of developing cardiovascular disease.
Historical Perspective
PCSK9 was initially described as neural apoptosis-regulated convertase-1 (NARC-1), which is expressed in cells that have the capacity to proliferate and differentiate such as hepatocytes, kidney mesenchymal cells, colon epithelial cells, and embryonic brain telencephalon neurons.[1] The function of PCSK9 was first described in 2003 when a gain-of-function mutation of PCSK9 gene (leading to increased activity) was associated with familial hypercholesterolemia in 4 french families.[2] The association was further clarified in 2005 after the discovery of loss-of-function mutations of PCSK9 in patients with low LDL-C. This loss-of-function was linked to a 40% reduction in plasma levels of LDL-C in the studied population.[3]
Biochemistry
Structure
Proprotein convertase subtilisin/kexin type 9 (PCSK9) is a serine protease encoded by the PCSK9 gene in humans.[4] PCSK9 is a 692 amino acid protein that is expressed mainly in the liver, intestine, and kidney.[5] PCSK9 gene encodes a proprotein convertase belonging to the proteinase K subfamily of the secretory subtilase family. The encoded protein is synthesized as a soluble zymogen that undergoes autocatalytic intramolecular processing in the endoplasmic reticulum. The protein may function as a proprotein convertase, and also plays a major regulatory role in cholesterol homeostasis.
Regulation
PCSK9 and LDL receptors are both mainly regulated by the transcription factor sterol-responsive element-binding protein 2 (SREBP2). SREBP2 is involved in a pathway also induced by statins[6] and by experimental resistin[7] which is an adipose-tissue derived adipokine. Another regulator of the PCSK9 gene expression is the hepatic nuclear factor 1 alpha (HNF1a), a transcription factor activated in the liver cells.[8]
Physiologic Function
Lipid Homeostasis
PCSK9 plays a major role in the metabolism of cholesterol. It binds to the epidermal growth factor-like repeat A (EGF-A) domain of the low-density lipoprotein receptor (LDLR), inducing LDLR endocytosis and degradation in lysosomes. Reduced LDL receptor levels result in decreased metabolism of low density lipoprotein (LDL) and increased levels of circulating LDL.[9] The sterol regulatory element-binding protein-2 (SREBP-2), which is activated in the presence of low intracellular levels of cholesterol, induces the expression of PCSK9. This leads to a decrease in LDL cholesterol metabolism thereby restoring normal levels of circulating.[10]
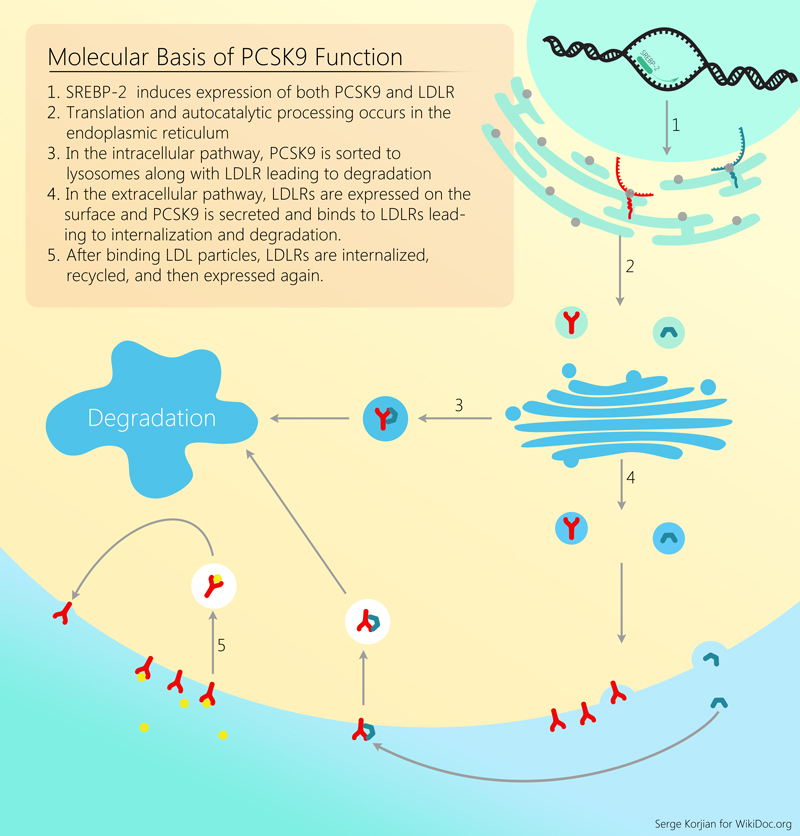
In addition to lowering LDL-C, PCSK9 deficiency has also been shown to lower cardiovascular risk factors by reducing postprandial hypertriglyceridemia.[12] PCSK9-deficient mice have also been demonstrated to have reduced lymphatic apoB secretion (the major lipoprotein of chylomicrons and LDL) as well as an increased ability to clear chylomicrons.[13]
Inflammation
PCSK9 is also an acute phase reactant whose expression increases in inflammatory states. The administration of lipopolysaccharide (LPS), an isolated bacterial protein that mimics acute infection or acute systemic inflammation, resulted in a 2.5-fold increase in PCSK9 mRNA levels and an increased PCSK9 expression in kidney tissues in mice.[14] In parallel, previous animal models have shown that LPS administration also produces an approximately 17-fold increase in LDL content of lysolecithin, a product derived from the oxidation of LDL.[15] These models have also been supported by studies showing strong association between inflammation and atherosclerosis in mice and hamsters. Although robust clinical data is still lacking, observational studies have shown an increased risk of coronary artery disease in patients with chronic inflammatory disorders. Furthermore, increased inflammatory markers are associated with adverse outcomes in patients with acute coronary syndromes. [16]
Apoptosis
Apoptotic cell death is one of the mechanisms implicated in the development of atherosclerosis. Oxidized LDL-induced apoptosis of human endothelial cells has been associated with an increased expression of PCSK9. Pretreatment of human endothelial cells with PCSK9-SiRNA (to inhibit PCSK9 expression) decreased LDL-induced apoptosis by reducing important mediators of apoptosis. PCSK9 reduced the Bcl-2/Bax ratio and inhibited the activation of both caspase 9 and 3.[17]
Blood Pressure Regulation
The epithelial Na+ channel (ENaC) regulates sodium homeostasis and plays a regulatory role in blood pressure control. It is a constitutively active ion-channel in the distal nephron responsible for active sodium reabsorption. Defects in ENaC are associated with essential forms of hereditary hypertension. PCSK9 was demonstrated to reduce ENaC protein expression in Xenopus epithelial cells by increasing endoplasmic reticulum-associated degradation and subsequently decreasing apical surface expression.[18]
Glucose Metabolism
Both PCSK9 and LDLR are expressed in insulin-producing pancreatic islet beta cells, and may be involved in the regulation of blood glucose. PCSK9-deficient mice were demonstrated to be hypoinsulinemic, hyperglycemic, and glucose-intolerant. Their islet cells exhibited signs of malformation, apoptosis and inflammation.[19] Nevertheless, the true effect of PCSK9 inhibition on glucose metabolism is unclear. The inhibition of PCSK9 by monoclonal antibodies had no significant effect on blood glucose and was not associated with worsening glycemic control in patients with diabetes.[20]
Adipose Tissue Metabolism
PCSK9-deficient mice were demonstrated to have adipocyte hypertrophy, increased in-vivo fatty acid uptake, and in-vitro triglyceride synthesis independent of LDL-receptors. Additionally, there was a 40-fold increase in cell surface levels of very-low-density lipoprotein receptors (VLDLR).[21] However, inhibition of PCSK9 by monoclonal antibodies was not demonstrated to increase central obesity.
PCSK9 Inhibition
Elevated LDL cholesterol levels in the plasma have previously been associated with the development and progression of atherosclerosis, as well as an increased risk of myocardial infarction and stroke. LDL receptors, which are responsible for clearing LDL cholesterol from the circulation, get recycled back into the plasma membrane in order to bind more LDL. A novel approach to the management of dyslipidemia targets the inhibition of the serine protease PCSK9 leading to increased LDL receptor expression and increased LDL cholesterol clearance. [22][23][24][25]
Natural
- Annexin A2 (AnxA2) is an endogenous compound that binds to the C-terminal domain of PCSK9 thereby preventing the interaction of PCSK9 with the LDL receptors particularly in the extrahepatic tissues. It has been demonstrated to be a functional inhibitor of PCSK9.[26]
- Furin and PC5/6A are two proprotein convertases that cause proteolytic cleavage of the PCSK9 protein between the R218 and Q219 residues resulting in a defective enzyme. Furin was demonstrated to regulate PCSK9 mRNA levels in hepatocytes.[27]
Pharmacologic
Several drugs have been investigated for the inhibition of PCSK9, and have demonstrated a more potent lowering of LDL cholesterol levels than the current available drugs. It is biologically plausible that this reduction in LDL would also lead to a reduction in atherothrombotic events. Initial human trials have demonstrated good tolerability and efficacy in lowering LDL choleterol, but additional phase III clinical trials are ongoing to demonstrate the effect of PCSK9 inhibition on cardiovascular events and outcomes.[22][23][24][25]
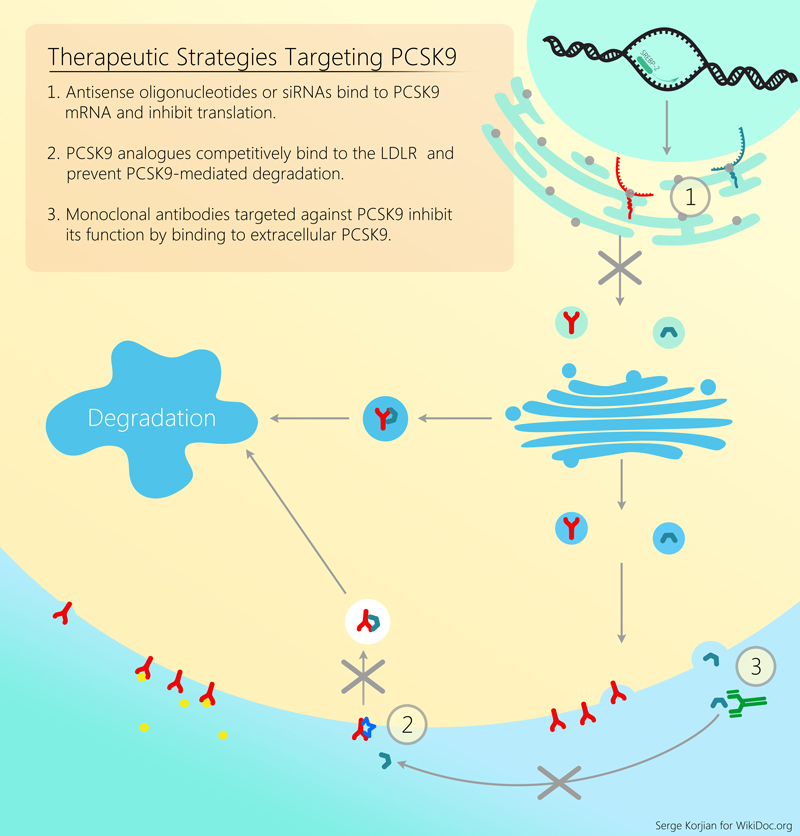
Monoclonal Antibodies
A number of monoclonal antibodies that bind to PCSK9 near the catalytic domain that interact with the LDL receptors, and hence inhibit the function of PCSK9 are currently in clinical trials. These include:
AMG 145 or Evolocumab by Amgen pharmaceuticals is a human monoclonal IgG2 antibody against PCSK9.
- RUTHERFORD trial - A multicenter, double-blinded, randomized, placebo-controlled, dose-ranging study to determine the efficacy and safety of evolocumab in heterozygous familial hypercholesterolemia patients. 168 patients receiving statins with or without ezetimibe were randomly assigned to subcutaneous evolocumab 350 mg, AMG 145 420 mg, or placebo administered every 4 weeks. At 12 weeks, LDL cholesterol was lowered by 43% and 55% with evolocumab 350 mg and 420 mg, respectively, compared with a 1% increase in the placebo group.[28]
- GAUSS trial - Based on the fact that approximately 10% to 20% of patients cannot tolerate statins, the GAUSS trial was designed to assess the efficacy and tolerability of evolocumab in patients with statin intolerance due to muscle-related side effects. 160 patients with statin intolerance were randomized equally into 5 different groups: evolocumab alone at 280 mg, 350 mg, or 420 mg doses; evolocumab at 420 mg plus 10 mg of ezetimibe and 10 mg of ezetimibe plus placebo - all given subcutaneously. At week 12, mean LDL cholesterol levels were lowered by 41% in the lowest dose group, 51% in the highest dose group, and 63% in the high dose group of evolocumab combined with ezetimive. In comparison, 15 the placebo/ezetimibe group demonstrated a 15% decrease in LDL cholesterol. Four serious adverse events were reported with evolocumab which were coronary artery disease, acute pancreatitis, hip fracture, syncope. Myalgia was also the most common treatment-emergent adverse effect observed during the study.[29]
- LAPLACE-TIMI 57 trial was designed to assess the efficacy, safety and tolerability to a range of doses of evolocumab in hypercholesterolemic patients. 631 patients on a stable dose of a statin (with or without ezetimibe) were randomly assigned to evolocumab at 70, 105, or 140 mg or placebo every two weeks or evolocumab at 280, 350, or 420 mg or placebo every four weeks. At week 12, mean LDL-C concentrations in the 2-week-dosing group was reduced from 42% to 66 % compared to a 42% to 50% reduction in the 4-week-dosing group. No serious or life-threatening events was observed.
- MENDEL trial was designed to assess the efficacy, safety and tolerability of evolocumab as monotherapy for hypercholesterolemia. 406 untreated hypercholesterolemic patients were assigned to similar groups as in the LAPLACE-TIMI 57 study and after 12 weeks, similar results with the prior studies were obtained (39 to 51% reduction in LDL cholesterol).[30]
- OSLER trial was an open label that included patients from any of the 4 phase II trials of evolocumab. It was designed to assess the efficacy and safety of longer-term (52-weeks) administration of evolocumabin patients with hypercholesterolemia. Of the initial patients enrolled in previous trials, 1104 patients (81%) elected to enroll into the OSLER study. Irrespective of initial treatment group, patients were randomized 2:1 to receive either standard of care with evolocumab 420 mg every 4 weeks or standard of care alone. Patients who received evolocumab demonstarted a 52.3% reduction in LDL choleterol levels, while patients who discontinued evolocumab and received standard of care in this trial had a return to baseline LDL levels by 12 weeks with no rebound effect. Adverse events occurred in 73.1% of patients in the standard of care arm compared to 81.4% of patients in the evolocumab arm.[20]
SAR236553/REGN727 or Alirocumab by Sanofi-Aventis/Regeneron pharmaceuticals[31]
- In a study to determine the safety and efficacy of alirocumab in patients with primary hypercholesterolemia receiving ongoing stable atorvastatin therapy, 183 patients with LDL-C≥100 mg/dl (2.59 mmol/l) on stable-dose atorvastatin 10, 20, or 40 mg for≥6 weeks were assigned to subcutaneous injections of alirocumab at 50, 100, or 150 mg doses every 2 weeks, alirocumab 200 or 300 mg every 4 weeks, or placebo every 2 weeks. After 12 weeks, LDL cholesterol levels were reduced by 40%, 64%, and 72% with 50, 100, and 150 mg in the 2-week-dosing group respectively, and 43% and 48% with 200 and 300 mg in the 4-week-dosing groups respectively. In comparison, patients in the placebo group had a 5% reduction in LDL cholesterol levels. Alirocumab was also found to reduce non-high-density lipoprotein cholesterol, apolipoprotein B, and lipoprotein(A). One case of leukocytoclastic vasculitis was reported.[32]
- ODYSSEY OUTCOMES is a phase III trial designed to evaluate the cardiovascular outcomes after an acute coronary syndrome in patients who have experienced an acute coronary syndrome (ACS) event 4 to 16 weeks prior to enrollment in the study. This trial is currently ongoing, but not recruiting participants.
RN316 or Bococizumab by Pfizer - In a study done by Pfizer to assess the safety and efficacy of RN316, intravenous doses of RN316 was shown to reduce the levels of LDL-C by 46% to 56% in patients already taking high-dose statins.
1D05-IgG2 by Merck & Co. - This is a PCSK9-binding antibody that structurally mimics the EGF(A) domain of LDL-receptor thereby reducing LDL cholesterol. It is currently under investigation. [33]
Other drugs being evaluated in phase I or II clinical trials include:
- RG7652 by Roche/Genentech
- LGT-209 by Novartis
- 1B20 by Merck & Co.
- J10, J16, J17 by Pfizer
Gene Silencing
Several agents work by shutting down the gene responsible for the synthesis of the PCSK9 protein.
- PCSK9 antisense oligonucleotide (ISIS 394814) from Isis Pharmaceuticals has been demonstrated to increase the expression of the LDL receptors and decrease circulating total cholesterol levels in mice.[34]
- Locked nucleic acids such as SPC4061 from Santaris Pharma demonstrated reduced PCSK9 mRNA levels when administered in mice.[35][36]
- ALN-PCS by The Medicines Company and Alnylam Pharmaceuticals acts by means of RNA interference, which causes the gene to shut down production of the PCSK9 protein.[37][38] Two drugs are being tested: ALN-PCS02 administered intravenously and ALN-PCSsc administered subcutaneously.
Mimetic Peptides
PCSK9 binds to the epidermal growth factor-like repeat A (EGF-A) domain of LDLR in order to induce its internalization and degradation. A mimetic peptide, which mimic the actions of EGF-A, was demonstrated to competitively inhibits PCSK9-mediated degradation of LDLR in HepG2 cells.[39] Examples of mimetic peptides currently being investigated include:
- EGF-AB peptide fragment by Schering-Plough
- LDLR (H306Y) subfragment by U.S. National Institute of Health
- LDLR DNA construct by U.S. National Institute of Health
AdnectinsTM
These are genetically engineered target-bindng proteins designed to bind specifically to therapeutic targets. They are similar to monoclonal antibodies including binding to targets with similar affinity and specificity, but differ in terms of sequence and lack of disulphide bonds in their single-domain structure.[40] The adnectin BMS-962476 by Bristol-Myers Squibb/Adnexus is currently in phase 1 clinical trial.
Small-Molecule Inhibitors
Orally administered small-molecule inhibitor could be a promising approach to PCSK9 inhibition, but their development has been challenging. First, small-molecule inhibitors might alter the sequence of PCSK9 auto-catalytic intracellular processing, secretion, and LDL receptor interaction.[41] Second, it has been also difficult to design a molecule that affects the flat and large target site of PCSK9 for LDLR.[42] Some small-molecule inhibitors under pre-clinical studies are:
- SX-PCK9 by Serometrix
- TBD by Shifa Biomedical
Clinical Significance of PCSK9 Inhibition
With the discovery of the PCSK9, many convincing studies and trials have reported the clinical efficacy and safety of the novel approaches to PCSK9 inhibition in patients either intolerant to statins or in those who failed to reach target LDL-C levels even at high doses of statins. However, certain questions regarding the long-term safety are still left unanswered. First is the issue of immunogenicity. Monoclonal antibodies against PCSK9 may elicit immune-mediated responses. This may be reduced with the use of fully human monoclonal antibodies.[43] This also underscores the requirement for a long term surveillance for antidrug antibodies in these patients. Secondly, most PCSK9 inhibitors are administered subcutaneously (few given intravenously) and they require 2 to 4-weekly dosing. This raises concern with regards to compliance, and efforts in search of an orally administered drug has been productive. Anacetrapid, a cholesterol ester transfer protein inhibitor has been reported to effectively lower LDL-C and increase HDL-C.[44] On the other hand, PCSK9 plays a role in triglyceride-rich lipoprotein metabolism. Statins have been shown to increase the serum levels of PCSK9, thus reducing their LDL-C lowering ability.[45][46] For this reason, a statin-PSCK9 inhibitor would have a synergistic effect in lowering the serum levels of LDL-C in the plasma. This has further shifted many effort towards this novel approach of PCSK9 inhibition as the next ultimate lipid modifier.
References
- ↑ Seidah, NG.; Benjannet, S.; Wickham, L.; Marcinkiewicz, J.; Jasmin, SB.; Stifani, S.; Basak, A.; Prat, A.; Chretien, M. (2003). "The secretory proprotein convertase neural apoptosis-regulated convertase 1 (NARC-1): liver regeneration and neuronal differentiation". Proc Natl Acad Sci U S A. 100 (3): 928–33. doi:10.1073/pnas.0335507100. PMID 12552133. Unknown parameter
|month=ignored (help) - ↑ Abifadel M, Varret M, Rabès JP, Allard D, Ouguerram K, Devillers M, Cruaud C, Benjannet S, Wickham L, Erlich D, Derré A, Villéger L, Farnier M, Beucler I, Bruckert E, Chambaz J, Chanu B, Lecerf JM, Luc G, Moulin P, Weissenbach J, Prat A, Krempf M, Junien C, Seidah NG, Boileau C (2003). "Mutations in PCSK9 cause autosomal dominant hypercholesterolemia". Nat. Genet. 34 (2): 154–6. doi:10.1038/ng1161. PMID 12730697. Unknown parameter
|month=ignored (help) - ↑ Cohen, J.; Pertsemlidis, A.; Kotowski, IK.; Graham, R.; Garcia, CK.; Hobbs, HH. (2005). "Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9". Nat Genet. 37 (2): 161–5. doi:10.1038/ng1509. PMID 15654334. Unknown parameter
|month=ignored (help) - ↑ Seidah NG, Benjannet S, Wickham L, Marcinkiewicz J, Jasmin SB, Stifani S, Basak A, Prat A, Chretien M (2003). "The secretory proprotein convertase neural apoptosis-regulated convertase 1 (NARC-1): liver regeneration and neuronal differentiation". Proc. Natl. Acad. Sci. U.S.A. 100 (3): 928–33. doi:10.1073/pnas.0335507100. PMC 298703. PMID 12552133. Unknown parameter
|month=ignored (help) - ↑ Zaid, A.; Roubtsova, A.; Essalmani, R.; Marcinkiewicz, J.; Chamberland, A.; Hamelin, J.; Tremblay, M.; Jacques, H.; Jin, W. (2008). "Proprotein convertase subtilisin/kexin type 9 (PCSK9): hepatocyte-specific low-density lipoprotein receptor degradation and critical role in mouse liver regeneration". Hepatology. 48 (2): 646–54. doi:10.1002/hep.22354. PMID 18666258. Unknown parameter
|month=ignored (help) - ↑ Lambert, G. (2007). "Unravelling the functional significance of PCSK9". Curr Opin Lipidol. 18 (3): 304–9. doi:10.1097/MOL.0b013e3281338531. PMID 17495605. Unknown parameter
|month=ignored (help) - ↑ Melone, M.; Wilsie, L.; Palyha, O.; Strack, A.; Rashid, S. (2012). "Discovery of a new role of human resistin in hepatocyte low-density lipoprotein receptor suppression mediated in part by proprotein convertase subtilisin/kexin type 9". J Am Coll Cardiol. 59 (19): 1697–705. doi:10.1016/j.jacc.2011.11.064. PMID 22554600. Unknown parameter
|month=ignored (help) - ↑ Dong, B.; Wu, M.; Li, H.; Kraemer, FB.; Adeli, K.; Seidah, NG.; Park, SW.; Liu, J. (2010). "Strong induction of PCSK9 gene expression through HNF1alpha and SREBP2: mechanism for the resistance to LDL-cholesterol lowering effect of statins in dyslipidemic hamsters". J Lipid Res. 51 (6): 1486–95. doi:10.1194/jlr.M003566. PMID 20048381. Unknown parameter
|month=ignored (help) - ↑ *"The Evolving Role of PCSK9 Modulation in the Regulation of LDL-Cholesterol". 2012-11-11.
- ↑ Maxwell, KN.; Soccio, RE.; Duncan, EM.; Sehayek, E.; Breslow, JL. (2003). "Novel putative SREBP and LXR target genes identified by microarray analysis in liver of cholesterol-fed mice". J Lipid Res. 44 (11): 2109–19. doi:10.1194/jlr.M300203-JLR200. PMID 12897189. Unknown parameter
|month=ignored (help) - ↑ 11.0 11.1 Urban, D.; Pöss, J.; Böhm, M.; Laufs, U. (2013). "Targeting the proprotein convertase subtilisin/kexin type 9 for the treatment of dyslipidemia and atherosclerosis". J Am Coll Cardiol. 62 (16): 1401–8. doi:10.1016/j.jacc.2013.07.056. PMID 23973703. Unknown parameter
|month=ignored (help) - ↑ Le May, C.; Kourimate, S.; Langhi, C.; Chétiveaux, M.; Jarry, A.; Comera, C.; Collet, X.; Kuipers, F.; Krempf, M. (2009). "Proprotein convertase subtilisin kexin type 9 null mice are protected from postprandial triglyceridemia". Arterioscler Thromb Vasc Biol. 29 (5): 684–90. doi:10.1161/ATVBAHA.108.181586. PMID 19265033. Unknown parameter
|month=ignored (help) - ↑ Sun, H.; Samarghandi, A.; Zhang, N.; Yao, Z.; Xiong, M.; Teng, BB. (2012). "Proprotein convertase subtilisin/kexin type 9 interacts with apolipoprotein B and prevents its intracellular degradation, irrespective of the low-density lipoprotein receptor". Arterioscler Thromb Vasc Biol. 32 (7): 1585–95. doi:10.1161/ATVBAHA.112.250043. PMID 22580899. Unknown parameter
|month=ignored (help) - ↑ Feingold, KR.; Moser, AH.; Shigenaga, JK.; Patzek, SM.; Grunfeld, C. (2008). "Inflammation stimulates the expression of PCSK9". Biochem Biophys Res Commun. 374 (2): 341–4. doi:10.1016/j.bbrc.2008.07.023. PMID 18638454. Unknown parameter
|month=ignored (help) - ↑ Memon RA, Staprans I, Noor M, Holleran WM, Uchida Y, Moser AH; et al. (2000). "Infection and inflammation induce LDL oxidation in vivo". Arterioscler Thromb Vasc Biol. 20 (6): 1536–42. PMID 10845869.
- ↑ Libby P, Ridker PM, Maseri A (2002). "Inflammation and atherosclerosis". Circulation. 105 (9): 1135–43. PMID 11877368.
- ↑ Wu, CY.; Tang, ZH.; Jiang, L.; Li, XF.; Jiang, ZS.; Liu, LS. (2012). "PCSK9 siRNA inhibits HUVEC apoptosis induced by ox-LDL via Bcl/Bax-caspase9-caspase3 pathway". Mol Cell Biochem. 359 (1–2): 347–58. doi:10.1007/s11010-011-1028-6. PMID 21847580. Unknown parameter
|month=ignored (help) - ↑ Sharotri, V.; Collier, DM.; Olson, DR.; Zhou, R.; Snyder, PM. (2012). "Regulation of epithelial sodium channel trafficking by proprotein convertase subtilisin/kexin type 9 (PCSK9)". J Biol Chem. 287 (23): 19266–74. doi:10.1074/jbc.M112.363382. PMID 22493497. Unknown parameter
|month=ignored (help) - ↑ Mbikay, M.; Sirois, F.; Mayne, J.; Wang, GS.; Chen, A.; Dewpura, T.; Prat, A.; Seidah, NG.; Chretien, M. (2010). "PCSK9-deficient mice exhibit impaired glucose tolerance and pancreatic islet abnormalities". FEBS Lett. 584 (4): 701–6. doi:10.1016/j.febslet.2009.12.018. PMID 20026049. Unknown parameter
|month=ignored (help) - ↑ 20.0 20.1 Mearns BM (2014). "Dyslipidaemia: 1-Year results from OSLER trial of anti-PCSK9 monoclonal antibody evolocumab". Nat Rev Cardiol. 11 (2): 63. doi:10.1038/nrcardio.2013.201. PMID 24322554.
- ↑ Roubtsova, A.; Munkonda, MN.; Awan, Z.; Marcinkiewicz, J.; Chamberland, A.; Lazure, C.; Cianflone, K.; Seidah, NG.; Prat, A. (2011). "Circulating proprotein convertase subtilisin/kexin 9 (PCSK9) regulates VLDLR protein and triglyceride accumulation in visceral adipose tissue". Arterioscler Thromb Vasc Biol. 31 (4): 785–91. doi:10.1161/ATVBAHA.110.220988. PMID 21273557. Unknown parameter
|month=ignored (help) - ↑ 22.0 22.1 Lopez D (2008). "Inhibition of PCSK9 as a novel strategy for the treatment of hypercholesterolemia". Drug News Perspect. 21 (6): 323–30. doi:10.1358/dnp.2008.21.6.1246795. PMID 18836590.
- ↑ 23.0 23.1 Steinberg D, Witztum JL (2009). "Inhibition of PCSK9: a powerful weapon for achieving ideal LDL cholesterol levels". Proc. Natl. Acad. Sci. U.S.A. 106 (24): 9546–7. doi:10.1073/pnas.0904560106. PMC 2701045. PMID 19506257. Unknown parameter
|month=ignored (help) - ↑ 24.0 24.1 Mayer G, Poirier S, Seidah NG (2008). "Annexin A2 is a C-terminal PCSK9-binding protein that regulates endogenous low density lipoprotein receptor levels". J. Biol. Chem. 283 (46): 31791–801. doi:10.1074/jbc.M805971200. PMID 18799458. Unknown parameter
|month=ignored (help) - ↑ 25.0 25.1 "Bristol-Myers Squibb selects Isis drug targeting PCSK9 as development candidate for prevention and treatment of cardiovascular disease". Press Release. FierceBiotech. 2008-04-08. Retrieved 2010-09-18.
- ↑ Seidah, NG.; Poirier, S.; Denis, M.; Parker, R.; Miao, B.; Mapelli, C.; Prat, A.; Wassef, H.; Davignon, J. (2012). "Annexin A2 is a natural extrahepatic inhibitor of the PCSK9-induced LDL receptor degradation". PLoS One. 7 (7): e41865. doi:10.1371/journal.pone.0041865. PMID 22848640.
- ↑ Essalmani R, Susan-Resiga D, Chamberland A, Abifadel M, Creemers JW, Boileau C; et al. (2011). "In vivo evidence that furin from hepatocytes inactivates PCSK9". J Biol Chem. 286 (6): 4257–63. doi:10.1074/jbc.M110.192104. PMC 3039354. PMID 21147780.
- ↑ Raal, F.; Scott, R.; Somaratne, R.; Bridges, I.; Li, G.; Wasserman, SM.; Stein, EA. (2012). "Low-density lipoprotein cholesterol-lowering effects of AMG 145, a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease in patients with heterozygous familial hypercholesterolemia: the Reduction of LDL-C with PCSK9 Inhibition in Heterozygous Familial Hypercholesterolemia Disorder (RUTHERFORD) randomized trial". Circulation. 126 (20): 2408–17. doi:10.1161/CIRCULATIONAHA.112.144055. PMID 23129602. Unknown parameter
|month=ignored (help) - ↑ Sullivan, D.; Olsson, AG.; Scott, R.; Kim, JB.; Xue, A.; Gebski, V.; Wasserman, SM.; Stein, EA. (2012). "Effect of a monoclonal antibody to PCSK9 on low-density lipoprotein cholesterol levels in statin-intolerant patients: the GAUSS randomized trial". JAMA. 308 (23): 2497–506. doi:10.1001/jama.2012.25790. PMID 23128163. Unknown parameter
|month=ignored (help) - ↑ Koren MJ, Scott R, Kim JB, Knusel B, Liu T, Lei L; et al. (2012). "Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 as monotherapy in patients with hypercholesterolaemia (MENDEL): a randomised, double-blind, placebo-controlled, phase 2 study". Lancet. 380 (9858): 1995–2006. doi:10.1016/S0140-6736(12)61771-1. PMID 23141812.
- ↑ Lambert G, Sjouke B, Choque B, Kastelein JJ, Hovingh GK (2012). "The PCSK9 decade". J. Lipid Res. 53 (12): 2515–24. doi:10.1194/jlr.R026658. PMC 3494258. PMID 22811413. Unknown parameter
|month=ignored (help) - ↑ McKenney JM, Koren MJ, Kereiakes DJ, Hanotin C, Ferrand AC, Stein EA (2012). "Safety and efficacy of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease, SAR236553/REGN727, in patients with primary hypercholesterolemia receiving ongoing stable atorvastatin therapy". J Am Coll Cardiol. 59 (25): 2344–53. doi:10.1016/j.jacc.2012.03.007. PMID 22463922.
- ↑ Ni YG, Di Marco S, Condra JH, Peterson LB, Wang W, Wang F; et al. (2011). "A PCSK9-binding antibody that structurally mimics the EGF(A) domain of LDL-receptor reduces LDL cholesterol in vivo". J Lipid Res. 52 (1): 78–86. doi:10.1194/jlr.M011445. PMC 2999929. PMID 20959675.
- ↑ Graham MJ, Lemonidis KM, Whipple CP, Subramaniam A, Monia BP, Crooke ST, Crooke RM (2007). "Antisense inhibition of proprotein convertase subtilisin/kexin type 9 reduces serum LDL in hyperlipidemic mice". J. Lipid Res. 48 (4): 763–7. doi:10.1194/jlr.C600025-JLR200. PMID 17242417. Unknown parameter
|month=ignored (help) - ↑ Gupta N, Fisker N, Asselin MC, Lindholm M, Rosenbohm C, Ørum H, Elmén J, Seidah NG, Straarup EM (2010). Deb, Sumitra, ed. "A locked nucleic acid antisense oligonucleotide (LNA) silences PCSK9 and enhances LDLR expression in vitro and in vivo". PLoS ONE. 5 (5): e10682. doi:10.1371/journal.pone.0010682. PMC 2871785. PMID 20498851.
- ↑ Lindholm MW, Elmén J, Fisker N, Hansen HF, Persson R, Møller MR, Rosenbohm C, Ørum H, Straarup EM, Koch T (2012). "PCSK9 LNA antisense oligonucleotides induce sustained reduction of LDL cholesterol in nonhuman primates". Mol. Ther. 20 (2): 376–81. doi:10.1038/mt.2011.260. PMC 3277239. PMID 22108858. Unknown parameter
|month=ignored (help) - ↑ "Alnylam Reports Positive Preliminary Clinical Results for ALN-PCS, an RNAi Therapeutic Targeting PCSK9 for the Treatment of Severe Hypercholesterolemia". Press Release. BusinessWire. 2011-01-04. Retrieved 2011-01-04.
- ↑ Frank-Kamenetsky M, Grefhorst A, Anderson NN, Racie TS, Bramlage B, Akinc A, Butler D, Charisse K, Dorkin R, Fan Y, Gamba-Vitalo C, Hadwiger P, Jayaraman M, John M, Jayaprakash KN, Maier M, Nechev L, Rajeev KG, Read T, Röhl I, Soutschek J, Tan P, Wong J, Wang G, Zimmermann T, de Fougerolles A, Vornlocher HP, Langer R, Anderson DG, Manoharan M, Koteliansky V, Horton JD, Fitzgerald K (2008). "Therapeutic RNAi targeting PCSK9 acutely lowers plasma cholesterol in rodents and LDL cholesterol in nonhuman primates". Proc. Natl. Acad. Sci. U.S.A. 105 (33): 11915–20. doi:10.1073/pnas.0805434105. PMC 2575310. PMID 18695239. Unknown parameter
|month=ignored (help) - ↑ Shan, L.; Pang, L.; Zhang, R.; Murgolo, NJ.; Lan, H.; Hedrick, JA. (2008). "PCSK9 binds to multiple receptors and can be functionally inhibited by an EGF-A peptide". Biochem Biophys Res Commun. 375 (1): 69–73. doi:10.1016/j.bbrc.2008.07.106. PMID 18675252. Unknown parameter
|month=ignored (help) - ↑ Lipovsek, D. (2011). "Adnectins: engineered target-binding protein therapeutics". Protein Eng Des Sel. 24 (1–2): 3–9. doi:10.1093/protein/gzq097. PMID 21068165. Unknown parameter
|month=ignored (help) - ↑ Do, RQ.; Vogel, RA.; Schwartz, GG. (2013). "PCSK9 Inhibitors: potential in cardiovascular therapeutics". Curr Cardiol Rep. 15 (3): 345. doi:10.1007/s11886-012-0345-z. PMID 23338726. Unknown parameter
|month=ignored (help) - ↑ Benjannet, S.; Hamelin, J.; Chrétien, M.; Seidah, NG. (2012). "Loss- and gain-of-function PCSK9 variants: cleavage specificity, dominant negative effects, and low density lipoprotein receptor (LDLR) degradation". J Biol Chem. 287 (40): 33745–55. doi:10.1074/jbc.M112.399725. PMID 22875854. Unknown parameter
|month=ignored (help) - ↑ Petrides F, Shearston K, Chatelais M, Guilbaud F, Meilhac O, Lambert G (2013). "The promises of PCSK9 inhibition". Curr Opin Lipidol. 24 (4): 307–12. doi:10.1097/MOL.0b013e328361f62d. PMID 23817198.
- ↑ Teramoto, T.; Shirakawa, M.; Kikuchi, M.; Nakagomi, M.; Tamura, S.; Surks, HK.; McCrary Sisk, C.; Numaguchi, H. (2013). "Efficacy and safety of the cholesteryl ester transfer protein inhibitor anacetrapib in Japanese patients with dyslipidemia". Atherosclerosis. 230 (1): 52–60. doi:10.1016/j.atherosclerosis.2013.05.012. PMID 23958252. Unknown parameter
|month=ignored (help) - ↑ Dubuc, G.; Chamberland, A.; Wassef, H.; Davignon, J.; Seidah, NG.; Bernier, L.; Prat, A. (2004). "Statins upregulate PCSK9, the gene encoding the proprotein convertase neural apoptosis-regulated convertase-1 implicated in familial hypercholesterolemia". Arterioscler Thromb Vasc Biol. 24 (8): 1454–9. doi:10.1161/01.ATV.0000134621.14315.43. PMID 15178557. Unknown parameter
|month=ignored (help) - ↑ Awan, Z.; Seidah, NG.; MacFadyen, JG.; Benjannet, S.; Chasman, DI.; Ridker, PM.; Genest, J. (2012). "Rosuvastatin, proprotein convertase subtilisin/kexin type 9 concentrations, and LDL cholesterol response: the JUPITER trial". Clin Chem. 58 (1): 183–9. doi:10.1373/clinchem.2011.172932. PMID 22065156. Unknown parameter
|month=ignored (help)