Mibefradil: Difference between revisions
No edit summary |
No edit summary |
||
| (3 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{Drugbox | {{Drugbox | ||
| Verifiedfields = changed | | Verifiedfields = changed | ||
| Line 19: | Line 15: | ||
<!--Pharmacokinetic data--> | <!--Pharmacokinetic data--> | ||
| bioavailability = | | bioavailability = 70 % | ||
| metabolism = | | protein_bound = > 99 % | ||
| elimination_half-life = | | metabolism = [[Hepatic]] | ||
| elimination_half-life = 17-25 h | |||
| excretion = | | excretion = | ||
| Line 52: | Line 49: | ||
| StdInChIKey_Ref = {{stdinchicite|changed|chemspider}} | | StdInChIKey_Ref = {{stdinchicite|changed|chemspider}} | ||
| StdInChIKey = HBNPJJILLOYFJU-VMPREFPWSA-N | | StdInChIKey = HBNPJJILLOYFJU-VMPREFPWSA-N | ||
| melting_point = 128 | |||
| melting_notes = (dihydrochloride salt) | |||
}} | }} | ||
__NOTOC__ | |||
{{SI}} | |||
{{CMG}} | |||
==Overview== | |||
'''Mibefradil''' ('''Posicor''') is a drug for the treatment of [[hypertension]] and chronic [[angina pectoris]]. It belongs to a group known as [[calcium channel blockers]]. | |||
It is nonselective.<ref name="pmid7565636">{{cite journal |author=Bezprozvanny I, Tsien RW |title=Voltage-dependent blockade of diverse types of voltage-gated Ca2+ channels expressed in Xenopus oocytes by the Ca2+ channel antagonist mibefradil (Ro 40-5967) |journal=Mol. Pharmacol. |volume=48 |issue=3 |pages=540–9 |date=September 1995 |pmid=7565636 |doi= |url=http://molpharm.aspetjournals.org/cgi/pmidlookup?view=long&pmid=7565636}}</ref> | It is nonselective.<ref name="pmid7565636">{{cite journal |author=Bezprozvanny I, Tsien RW |title=Voltage-dependent blockade of diverse types of voltage-gated Ca2+ channels expressed in Xenopus oocytes by the Ca2+ channel antagonist mibefradil (Ro 40-5967) |journal=Mol. Pharmacol. |volume=48 |issue=3 |pages=540–9 |date=September 1995 |pmid=7565636 |doi= |url=http://molpharm.aspetjournals.org/cgi/pmidlookup?view=long&pmid=7565636}}</ref> | ||
On June 8, 1998, [[Hoffmann–La Roche|Roche]] announced the voluntary withdrawal of the drug from the market, one year after approval by the FDA, due to the potential for drug interactions, some of them deadly, which may occur when it is taken together with some other medications. <ref>Heart Drug Withdrawn as Evidence Shows It Could Be Lethal: http://www.nytimes.com/1998/06/09/us/heart-drug-withdrawn-as-evidence-shows-it-could-be-lethal.html </ref> | On June 8, 1998, [[Hoffmann–La Roche|Roche]] announced the voluntary withdrawal of the drug from the market, one year after approval by the FDA, due to the potential for drug interactions, some of them deadly, which may occur when it is taken together with some other medications. <ref>Heart Drug Withdrawn as Evidence Shows It Could Be Lethal: http://www.nytimes.com/1998/06/09/us/heart-drug-withdrawn-as-evidence-shows-it-could-be-lethal.html </ref> | ||
==Synthesis== | |||
[[File:Mibefradil1.png|thumb|600px|center|Mibefradil synthesis]] | |||
==References== | ==References== | ||
{{Reflist|2}} | |||
{{Membrane transport modulators}} | {{Membrane transport modulators}} | ||
[[Category:Calcium channel blockers]][[Category:Withdrawn drugs]][[Category:Benzimidazoles]][[Category:Organofluorides]][[Category:Ethers]][[Category:Amines | [[Category:Calcium channel blockers]] | ||
[[Category:Withdrawn drugs]] | |||
[[Category:Benzimidazoles]] | |||
[[Category:Organofluorides]] | |||
[[Category:Ethers]] | |||
[[Category:Amines]] | |||
[[Category:Drug]] | |||
Latest revision as of 19:24, 8 April 2015
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| MedlinePlus | a607007 |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 70 % |
| Protein binding | > 99 % |
| Metabolism | Hepatic |
| Elimination half-life | 17-25 h |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
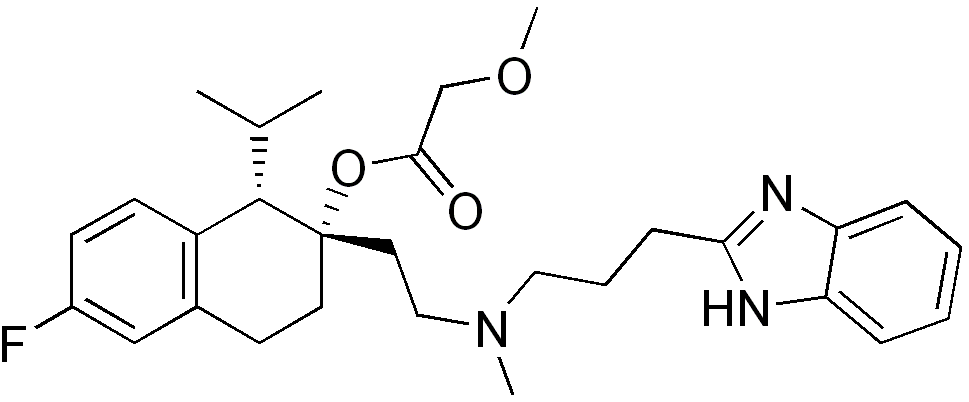
| Formula | C29H38FN3O3 |
| Molar mass | 495.63 g/mol |
| 3D model (JSmol) | |
| Melting point | 128 °C (262.4 °F) (dihydrochloride salt) |
| |
| |
| | |
|
WikiDoc Resources for Mibefradil |
|
Articles |
|---|
|
Most recent articles on Mibefradil |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Mibefradil at Clinical Trials.gov Clinical Trials on Mibefradil at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Mibefradil
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Mibefradil Discussion groups on Mibefradil Patient Handouts on Mibefradil Directions to Hospitals Treating Mibefradil Risk calculators and risk factors for Mibefradil
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Mibefradil |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Mibefradil (Posicor) is a drug for the treatment of hypertension and chronic angina pectoris. It belongs to a group known as calcium channel blockers.
It is nonselective.[1]
On June 8, 1998, Roche announced the voluntary withdrawal of the drug from the market, one year after approval by the FDA, due to the potential for drug interactions, some of them deadly, which may occur when it is taken together with some other medications. [2]
Synthesis
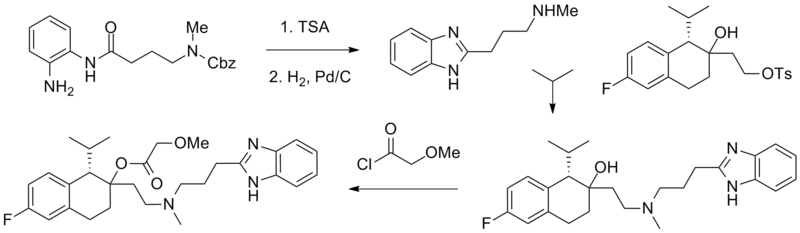
References
- ↑ Bezprozvanny I, Tsien RW (September 1995). "Voltage-dependent blockade of diverse types of voltage-gated Ca2+ channels expressed in Xenopus oocytes by the Ca2+ channel antagonist mibefradil (Ro 40-5967)". Mol. Pharmacol. 48 (3): 540–9. PMID 7565636.
- ↑ Heart Drug Withdrawn as Evidence Shows It Could Be Lethal: http://www.nytimes.com/1998/06/09/us/heart-drug-withdrawn-as-evidence-shows-it-could-be-lethal.html
- Pages with script errors
- Template:drugs.com link with non-standard subpage
- Drugs with non-standard legal status
- Articles with changed DrugBank identifier
- Articles with changed ChemSpider identifier
- Articles with changed EBI identifier
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles with changed InChI identifier
- Chemical articles with unknown parameter in Infobox drug
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- Calcium channel blockers
- Withdrawn drugs
- Benzimidazoles
- Organofluorides
- Ethers
- Amines
- Drug