Meprobamate: Difference between revisions
m (Protected "Meprobamate": Robot: Protecting all pages from category Drug ([edit=sysop] (indefinite) [move=sysop] (indefinite))) |
Kiran Singh (talk | contribs) No edit summary |
||
| (22 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{ | {{DrugProjectFormSinglePage | ||
|IUPAC_name = [2-(carbamoyloxymethyl)-2-methyl-pentyl] carbamate | |authorTag={{KS}} | ||
| image= | |genericName=meprobamate | ||
| image2 = | |aOrAn=an | ||
| | |drugClass=anti anxiety drug | ||
|indicationType=treatment | |||
| | |indication=[[anxiety disorders]] | ||
| | |adverseReactions=[[nausea]], [[vomiting]], [[diarrhea]]. [[drowsiness]], [[ataxia]], [[dizziness]], [[slurred speech]], [[headache]], [[vertigo]], [[weakness]], [[paresthesias]] | ||
| | |blackBoxWarningTitle=<span style="color:#FF0000;">ConditionName: </span> | ||
| | |blackBoxWarningBody=<i><span style="color:#FF0000;">ConditionName: </span></i> | ||
| | |||
| | * Content | ||
| bioavailability= ? | |||
<!--Adult Indications and Dosage--> | |||
<!--FDA-Labeled Indications and Dosage (Adult)--> | |||
|fdaLIADAdult===Indications== | |||
* Meprobamate tablets are indicated for the management of [[anxiety disorders]] or for the short-term relief of the symptoms of [[anxiety]]. [[Anxiety]] or tension associated with the stress of everyday life usually do not require treatment with an anxiolytic. | |||
* The effectiveness of meprobamate tablets in long-term use, that is, more than 4 months, has not been assessed by systematic clinical studies. The physician should periodically reassess the usefulness of the drug for the individual patient. | |||
==Dosage== | |||
* Meprobamate Tablets USP: The usual adult daily dosage is 1200mg to 1600 mg, in three or four divided doses; a daily dosage above 2400 mg is not recommended. | |||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
|offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
|fdaLIADPed===Indications== | |||
* Meprobamate tablets are indicated for the management of [[anxiety disorders]] or for the short-term relief of the symptoms of [[anxiety]]. [[Anxiety]] or tension associated with the stress of everyday life usually do not require treatment with an anxiolytic. | |||
* The effectiveness of meprobamate tablets in long-term use, that is, more than 4 months, has not been assessed by systematic clinical studies. The physician should periodically reassess the usefulness of the drug for the individual patient. | |||
==Dosing== | |||
* The usual daily dosage for children ages six to twelve years is 200 mg to 600 mg, in two or three divided doses. | |||
* Not recommended for children under age 6 (see Usage in Children). | |||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
|contraindications=* [[Acute intermittent porphyria]] as well as allergic or idiosyncratic reactions to meprobamate or related compounds such as carisoprodol, mebutamate, tybamate, or carbromal. | |||
|warnings='''Drug Dependence''' | |||
* Physical dependence, psychological dependence, and abuse have occurred. When chronic intoxication from prolonged use occurs, it usually involves ingestion of greater than recommended doses and is manifested by [[ataxia]], slurred speech, and [[vertigo]]. | |||
* Therefore, careful supervision of dose and amounts prescribed is advised, as well as avoidance of prolonged administration, especially for alcoholics and other patients with a known propensity for taking excessive quantities of drugs. | |||
* Sudden withdrawal of the drug after prolonged and excessive use may precipitate recurrence of pre-existing symptoms such as [[anxiety]], [[anorexia]], insomnia, or withdrawal reactions such as [[vomiting]], [[ataxia]], tremors, muscle twitching, confusional states, hallucinosis, and rarely, convulsive [[seizures]]. Such [[seizures]] are more likely to occur in persons with central nervous system damage or pre-existent or latent convulsive disorders. Onset of withdrawal symptoms occurs usually within 12 to 48 hours after discontinuation of meprobamate; symptoms usually cease within the next 12 to 48 hours. | |||
* When excessive dosage has continued for weeks or months, dosage should be reduced gradually over a period of one or two weeks rather than abruptly stopped. Alternatively, a long-acting barbiturate may be substituted, then gradually withdrawn. | |||
'''Potentially Hazardous Tasks''' | |||
* Patients should be warned that meprobamate may impair the mental and/or physical abilities required for performance of potentially hazardous tasks such as driving or operating machinery. | |||
'''Additive Effects''' | |||
* Since the effects of meprobamate and alcohol or meprobamate and other CNS depressants or psychotropic drugs may be additive, appropriate caution should be exercised with patients who take more than one of these agents simultaneously. | |||
'''Usage in Pregnancy and Lactation''' | |||
* An increased risk of congenital malformations associated with the use of minor tranquilizers (meprobamate, chlordiazepoxide and diazepam) during the first trimester of pregnancy has been suggested in several studies. Because use of these drugs is rarely a matter of urgency, their use during this period should almost always be avoided. The possibility that a woman of childbearing potential may be pregnant at the time of institution of therapy should be considered. Patients should be advised that if they become pregnant during therapy or intend to become pregnant they should communicate with their physician about the desirability of discontinuing the drug. | |||
* Meprobamate passes the placental barrier. It is present both in umbilical cord blood at or near maternal plasma levels and in breast milk of lactating mothers at concentrations two to four times that of maternal plasma. When use of meprobamate is contemplated in breastfeeding patients, the drug's higher concentration in breast milk as compared to maternal plasma should be considered. | |||
'''Usage in Children''' | |||
* Meprobamate tablets should not be administered to children under age six, since there is a lack of documented evidence for safety and effectiveness in this age group. | |||
==PRECAUTIONS== | |||
* The lowest effective dose should be administered, particularly to elderly and/or debilitated patients, in order to preclude oversedation. | |||
* The possibility of suicide attempts should be considered and the least amount of drug feasible should be dispensed at any one time. | |||
* Meprobamate is metabolized in the liver and excreted by the kidney; to avoid its excess accumulation, caution should be exercised in administration to patients with compromised liver or kidney function. | |||
* Meprobamate occasionally may precipitate [[seizures]] in epileptic patients. | |||
|clinicalTrials='''Central Nervous System''' | |||
* [[Drowsiness]], [[ataxia]], [[dizziness]], slurred speech, [[headache]], [[vertigo]], weakness, [[paresthesias]], impairment of visual accommodation, euphoria, overstimulation, paradoxical excitement, fast EEG activity. | |||
'''Gastrointestinal''' | |||
* [[Nausea]], [[vomiting]], [[diarrhea]]. | |||
'''Cardiovascular''' | |||
* [[Palpitation]], [[tachycardia]], various forms of [[arrhythmia]], transient ECG changes, [[syncope]]; also [[hypotensive]] crisis (including one fatal case). | |||
'''Allergic or Idiosyncratic''' | |||
* Allergic or idiosyncratic reactions are usually seen within the period of the first to fourth dose in patients having had no previous contact with the drug. Milder reactions are characterized by an [[itchy]], [[urticarial]], or erythematous maculopapular rash which may be generalized or confined to the groin. Other reactions have included [[leukopenia]], acute [[nonthrombocytopenic purpura]], [[petechiae]], [[ecchymoses]], eosinophilia, [[peripheral edema]], adenopathy, fever, fixed drug eruption with cross reaction to carisoprodol, and cross sensitivity between meprobamate/mebutamate and meprobamate/carbromal. | |||
* More severe hypersensitivity reactions, rarely reported, include hyperpyrexia [[chills]], [[angioneurotic edema]], [[bronchospasm]], [[oliguria]] and [[anuria]]. Also, [[anaphylaxis]], [[erythema multiforme]], [[exfoliative dermatitis]], [[stomatitis]], [[proctitis]], [[Stevens-Johnson syndrome]] and [[bullous dermatitis]], including one fatal case of the latter following administration of meprobamate in combination with prednisolone. | |||
* In case of allergic or idiosyncratic reactions to meprobamate, discontinue the drug and initiate appropriate symptomatic therapy, which may include epinephrine,antihistamines, and in severe cases, corticosteroids. In evaluating possible allergic reactions, also consider allergy to excipients. | |||
'''Hematologic''' | |||
* [[Agranulocytosis]] and [[aplastic anemia]] have been reported. These cases rarely were fatal. Rare cases of [[thrombocytopenic Purpura]] have been reported. | |||
'''Other''' | |||
* Exacerbation of porphyric symptoms. | |||
|postmarketing=There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | |||
|useInPregnancyAUS=* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | |||
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | |||
|useInLaborDelivery=There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | |||
|useInNursing=There is no FDA guidance on the use of {{PAGENAME}} with respect to nursing mothers. | |||
|useInPed=There is no FDA guidance on the use of {{PAGENAME}} with respect to pediatric patients. | |||
|useInGeri=* Clinical studies of meprobamate tablets did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. | |||
|useInGender=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | |||
|useInRace=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | |||
|useInRenalImpair=There is no FDA guidance on the use of {{PAGENAME}} in patients with renal impairment. | |||
|useInHepaticImpair=There is no FDA guidance on the use of {{PAGENAME}} in patients with hepatic impairment. | |||
|useInReproPotential=There is no FDA guidance on the use of {{PAGENAME}} in women of reproductive potentials and males. | |||
|useInImmunocomp=There is no FDA guidance one the use of {{PAGENAME}} in patients who are immunocompromised. | |||
|administration=* Oral | |||
|monitoring=There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | |||
<!--IV Compatibility--> | |||
|IVCompat=There is limited information regarding <i>IV Compatibility</i> of {{PAGENAME}} in the drug label. | |||
|overdose=* Suicidal attempts with meprobamate have resulted in drowsiness, lethargy, stupor, ataxia, coma, shock, vasomotor, and respiratory collapse. Some suicidal attempts have been fatal. | |||
* The following data on meprobamate tablets have been reported in the literature and from other sources. These data are not expected to correlate with each case (considering factors such as individual susceptibility and length of time from ingestion to treatment), but represent the usual ranges reported. | |||
'''Acute simple overdose''' (meprobamate alone): Death has been reported with ingestion of as little as 12 g meprobamate and survival with as much as 40 g. | |||
'''Blood Levels''' | |||
* 0.5-2 mg% represents the usual blood level range of meprobamate after therapeutic doses. The level may occasionally be as high as 3 mg%. | |||
* 3-10 mg% usually corresponds to findings of mild to moderate symptoms of overdosage, such as stupor or light coma. | |||
* 10-20 mg% usually corresponds to deeper coma, requiring more intensive treatment. Some fatalities occur. | |||
* At levels greater than 20 mg%, more fatalities than survivals can be expected. | |||
'''Acute combined overdose''' (meprobamate with alcohol or other CNS depressants or psychotropic drugs): Since effects can be additive, a history of ingestion of a low dose of meprobamate plus any of these compounds (or of a relative low blood or tissue level) cannot be used as a prognostic indicator. | |||
* In cases where excessive doses have been taken, sleep ensues rapidly and blood pressure, pulse, and respiratory rates are reduced to basal levels. Any drug remaining in the stomach should be removed and symptomatic therapy given. Should respiration or blood pressure become compromised, respiratory assistance, central nervous system stimulants, and pressor agents should be administered cautiously as indicated. Meprobamate is metabolized in the liver and excreted by the kidney. Diuresis, osmotic (mannitol) diuresis, peritoneal dialysis, and hemodialysis have been used successfully. Careful monitoring of urinary output is necessary and caution should be taken to avoid overhydration. Relapse and death, after initial recovery, have been attributed to incomplete gastric emptying and delayed absorption. Meprobamate can be measured in biological fluids by two methods: colorimetric (Hoffman, A.J. and Ludwig, B.J.: J Amer Pharm Assn 48: 740, 1959) and gas chromatographic (Douglas, J.F. et al: Anal Chem 39: 956, 1967). | |||
|drugBox={{Drugbox2 | |||
| Watchedfields = changed | |||
| verifiedrevid = 459508597 | |||
| IUPAC_name = [2-(carbamoyloxymethyl)-2-methyl-pentyl] carbamate | |||
| image = Meprobamate2DCSD.png | |||
| width = 230px | |||
| image2 = Meprobamate3DanJ.gif | |||
| width2 = 300px | |||
<!--Clinical data--> | |||
| tradename = Miltown | |||
| Drugs.com = {{drugs.com|monograph|meprobamate}} | |||
| MedlinePlus = a682077 | |||
| pregnancy_AU = C | |||
| pregnancy_US = D | |||
| legal_AU = S4 | |||
| legal_US = Schedule IV | |||
| legal_Ca = Schedule IV | |||
| routes_of_administration = Oral | |||
<!--Pharmacokinetic data--> | |||
| bioavailability = ? | |||
| metabolism = [[Liver|Hepatic]] | | metabolism = [[Liver|Hepatic]] | ||
| elimination_half-life= 10 hours | | elimination_half-life = 10 hours | ||
| excretion = [[Kidney|Renal]] | | excretion = [[Kidney|Renal]] | ||
== | <!--Identifiers--> | ||
| CASNo_Ref = {{cascite|correct|CAS}} | |||
| CAS_number_Ref = {{cascite|correct|??}} | |||
| CAS_number = 57-53-4 | |||
| ATC_prefix = N05 | |||
| ATC_suffix = BC01 | |||
| PubChem = 4064 | |||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| DrugBank = DB00371 | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 3924 | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = 9I7LNY769Q | |||
| KEGG_Ref = {{keggcite|correct|kegg}} | |||
| KEGG = D00376 | |||
| ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| ChEMBL = 979 | |||
<!--Chemical data--> | |||
| C=9 | H=18 | N=2 | O=4 | |||
| molecular_weight = 218.250 g/mol | |||
| smiles = O=C(OCC(COC(=O)N)(C)CCC)N | |||
| InChI = 1/C9H18N2O4/c1-3-4-9(2,5-14-7(10)12)6-15-8(11)13/h3-6H2,1-2H3,(H2,10,12)(H2,11,13) | |||
| InChIKey = NPPQSCRMBWNHMW-UHFFFAOYAM | |||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChI = 1S/C9H18N2O4/c1-3-4-9(2,5-14-7(10)12)6-15-8(11)13/h3-6H2,1-2H3,(H2,10,12)(H2,11,13) | |||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChIKey = NPPQSCRMBWNHMW-UHFFFAOYSA-N | |||
| density = 1.229 | |||
| melting_point = 105 | |||
| melting_high = 106 | |||
| boiling_point = 200 | |||
| boiling_notes = to 210 °C (410 °F) | |||
}} | |||
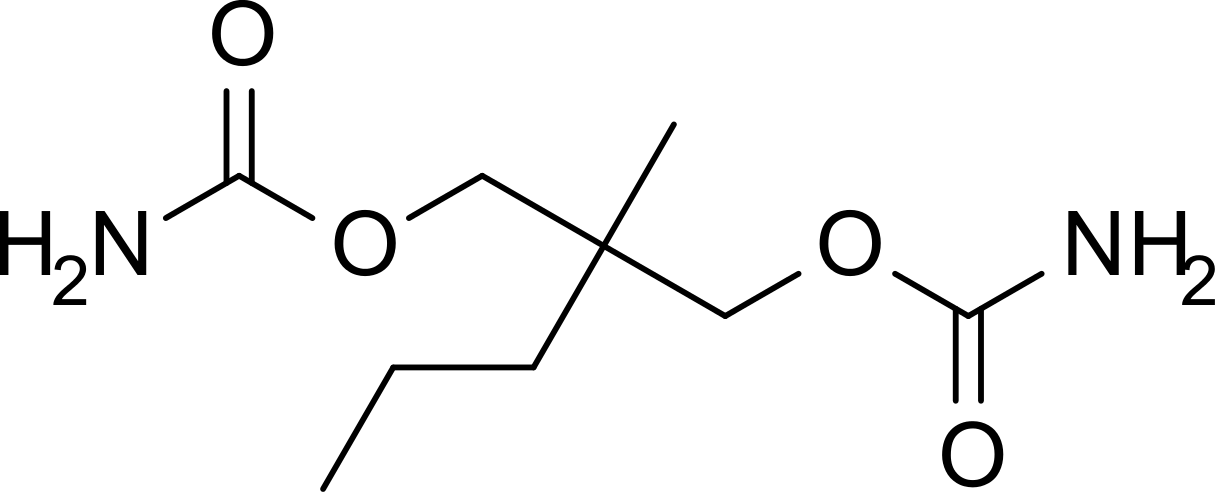
|structure=Meprobamate is a white powder with a characteristic odor and a bitter taste. It is slightly soluble in water, freely soluble in acetone and alcohol, and sparingly soluble in ether. The structural formula of meprobamate is: | |||
[[File:Meprobamate structure.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
Meprobamate Tablets USP 200 mg and 400 mg for oral administration contain the following inactive ingredients: colloidal silicon dioxide, magnesium stearate, microcrystalline cellulose, sodium starch glycolate and pregelatinised starch. | |||
|PD=There is limited information regarding <i>Pharmacodynamics</i> of {{PAGENAME}} in the drug label. | |||
|PK=There is limited information regarding <i>Pharmacokinetics</i> of {{PAGENAME}} in the drug label. | |||
|nonClinToxic=There is limited information regarding <i>Nonclinical Toxicology</i> of {{PAGENAME}} in the drug label. | |||
|clinicalStudies=There is limited information regarding <i>Clinical Studies</i> of {{PAGENAME}} in the drug label. | |||
|howSupplied=* Meprobamate Tablets USP 200 mg are white to off white, round, biconvex, uncoated tablets debossed with “L125” on one side and break line on other side. | |||
:*NDC 23155-128-03 Bottle of 30 | |||
:*NDC 23155-128-01 Bottle of 100 | |||
:*NDC 23155-128-10 Bottle of 1000 | |||
* Meprobamate Tablets USP 400 mg are white to off white, round, biconvex, uncoated tablets debossed with “L105” on one side and break line on other side. | |||
:*NDC 23155-129-03 Bottle of 30 | |||
:*NDC 23155-129-01 Bottle of 100 | |||
:*NDC 23155-129-10 Bottle of 1000 | |||
* Dispense in well-closed container with child-resistant closure. | |||
|storage=* Store at controlled room temperature, excursions permitted to 15°C-30°C (59°F-86°F). | |||
== | * Preserve in well closed container. | ||
|fdaPatientInfo=There is limited information regarding <i>Patient Counseling Information</i> of {{PAGENAME}} in the drug label. | |||
|alcohol=* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |||
|brandNames=MEPROBAMATE | |||
|drugShortage= | |||
}} | |||
{{PillImage | |||
|fileName=No image.jpg | |||
}} | |||
{{LabelImage | |||
|fileName=Meprobamate ingredients and appearance.png | |||
}} | |||
{{LabelImage | |||
|fileName=Meprobamate fig01.jpg | |||
}} | |||
{{LabelImage | |||
|fileName=Meprobamate fig.jpg | |||
}} | |||
<!--Pill Image--> | |||
<!--Label Display Image--> | |||
<!--Category--> | |||
[[Category:Sedatives]] | [[Category:Sedatives]] | ||
[[Category:Hypnotics]] | [[Category:Hypnotics]] | ||
[[Category:Muscle relaxants]] | [[Category:Muscle relaxants]] | ||
[[Category: | [[Category:Drug]] | ||
Latest revision as of 19:35, 9 March 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Kiran Singh, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Meprobamate is an anti anxiety drug that is FDA approved for the treatment of anxiety disorders. Common adverse reactions include nausea, vomiting, diarrhea. drowsiness, ataxia, dizziness, slurred speech, headache, vertigo, weakness, paresthesias.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- Meprobamate tablets are indicated for the management of anxiety disorders or for the short-term relief of the symptoms of anxiety. Anxiety or tension associated with the stress of everyday life usually do not require treatment with an anxiolytic.
- The effectiveness of meprobamate tablets in long-term use, that is, more than 4 months, has not been assessed by systematic clinical studies. The physician should periodically reassess the usefulness of the drug for the individual patient.
Dosage
- Meprobamate Tablets USP: The usual adult daily dosage is 1200mg to 1600 mg, in three or four divided doses; a daily dosage above 2400 mg is not recommended.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Meprobamate in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Meprobamate in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Indications
- Meprobamate tablets are indicated for the management of anxiety disorders or for the short-term relief of the symptoms of anxiety. Anxiety or tension associated with the stress of everyday life usually do not require treatment with an anxiolytic.
- The effectiveness of meprobamate tablets in long-term use, that is, more than 4 months, has not been assessed by systematic clinical studies. The physician should periodically reassess the usefulness of the drug for the individual patient.
Dosing
- The usual daily dosage for children ages six to twelve years is 200 mg to 600 mg, in two or three divided doses.
- Not recommended for children under age 6 (see Usage in Children).
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Meprobamate in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Meprobamate in pediatric patients.
Contraindications
- Acute intermittent porphyria as well as allergic or idiosyncratic reactions to meprobamate or related compounds such as carisoprodol, mebutamate, tybamate, or carbromal.
Warnings
Drug Dependence
- Physical dependence, psychological dependence, and abuse have occurred. When chronic intoxication from prolonged use occurs, it usually involves ingestion of greater than recommended doses and is manifested by ataxia, slurred speech, and vertigo.
- Therefore, careful supervision of dose and amounts prescribed is advised, as well as avoidance of prolonged administration, especially for alcoholics and other patients with a known propensity for taking excessive quantities of drugs.
- Sudden withdrawal of the drug after prolonged and excessive use may precipitate recurrence of pre-existing symptoms such as anxiety, anorexia, insomnia, or withdrawal reactions such as vomiting, ataxia, tremors, muscle twitching, confusional states, hallucinosis, and rarely, convulsive seizures. Such seizures are more likely to occur in persons with central nervous system damage or pre-existent or latent convulsive disorders. Onset of withdrawal symptoms occurs usually within 12 to 48 hours after discontinuation of meprobamate; symptoms usually cease within the next 12 to 48 hours.
- When excessive dosage has continued for weeks or months, dosage should be reduced gradually over a period of one or two weeks rather than abruptly stopped. Alternatively, a long-acting barbiturate may be substituted, then gradually withdrawn.
Potentially Hazardous Tasks
- Patients should be warned that meprobamate may impair the mental and/or physical abilities required for performance of potentially hazardous tasks such as driving or operating machinery.
Additive Effects
- Since the effects of meprobamate and alcohol or meprobamate and other CNS depressants or psychotropic drugs may be additive, appropriate caution should be exercised with patients who take more than one of these agents simultaneously.
Usage in Pregnancy and Lactation
- An increased risk of congenital malformations associated with the use of minor tranquilizers (meprobamate, chlordiazepoxide and diazepam) during the first trimester of pregnancy has been suggested in several studies. Because use of these drugs is rarely a matter of urgency, their use during this period should almost always be avoided. The possibility that a woman of childbearing potential may be pregnant at the time of institution of therapy should be considered. Patients should be advised that if they become pregnant during therapy or intend to become pregnant they should communicate with their physician about the desirability of discontinuing the drug.
- Meprobamate passes the placental barrier. It is present both in umbilical cord blood at or near maternal plasma levels and in breast milk of lactating mothers at concentrations two to four times that of maternal plasma. When use of meprobamate is contemplated in breastfeeding patients, the drug's higher concentration in breast milk as compared to maternal plasma should be considered.
Usage in Children
- Meprobamate tablets should not be administered to children under age six, since there is a lack of documented evidence for safety and effectiveness in this age group.
PRECAUTIONS
- The lowest effective dose should be administered, particularly to elderly and/or debilitated patients, in order to preclude oversedation.
- The possibility of suicide attempts should be considered and the least amount of drug feasible should be dispensed at any one time.
- Meprobamate is metabolized in the liver and excreted by the kidney; to avoid its excess accumulation, caution should be exercised in administration to patients with compromised liver or kidney function.
- Meprobamate occasionally may precipitate seizures in epileptic patients.
Adverse Reactions
Clinical Trials Experience
Central Nervous System
- Drowsiness, ataxia, dizziness, slurred speech, headache, vertigo, weakness, paresthesias, impairment of visual accommodation, euphoria, overstimulation, paradoxical excitement, fast EEG activity.
Gastrointestinal
Cardiovascular
- Palpitation, tachycardia, various forms of arrhythmia, transient ECG changes, syncope; also hypotensive crisis (including one fatal case).
Allergic or Idiosyncratic
- Allergic or idiosyncratic reactions are usually seen within the period of the first to fourth dose in patients having had no previous contact with the drug. Milder reactions are characterized by an itchy, urticarial, or erythematous maculopapular rash which may be generalized or confined to the groin. Other reactions have included leukopenia, acute nonthrombocytopenic purpura, petechiae, ecchymoses, eosinophilia, peripheral edema, adenopathy, fever, fixed drug eruption with cross reaction to carisoprodol, and cross sensitivity between meprobamate/mebutamate and meprobamate/carbromal.
- More severe hypersensitivity reactions, rarely reported, include hyperpyrexia chills, angioneurotic edema, bronchospasm, oliguria and anuria. Also, anaphylaxis, erythema multiforme, exfoliative dermatitis, stomatitis, proctitis, Stevens-Johnson syndrome and bullous dermatitis, including one fatal case of the latter following administration of meprobamate in combination with prednisolone.
- In case of allergic or idiosyncratic reactions to meprobamate, discontinue the drug and initiate appropriate symptomatic therapy, which may include epinephrine,antihistamines, and in severe cases, corticosteroids. In evaluating possible allergic reactions, also consider allergy to excipients.
Hematologic
- Agranulocytosis and aplastic anemia have been reported. These cases rarely were fatal. Rare cases of thrombocytopenic Purpura have been reported.
Other
- Exacerbation of porphyric symptoms.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Meprobamate in the drug label.
Drug Interactions
There is limited information regarding Meprobamate Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Meprobamate in women who are pregnant.
Pregnancy Category (AUS):
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Meprobamate in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Meprobamate during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Meprobamate with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Meprobamate with respect to pediatric patients.
Geriatic Use
- Clinical studies of meprobamate tablets did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Gender
There is no FDA guidance on the use of Meprobamate with respect to specific gender populations.
Race
There is no FDA guidance on the use of Meprobamate with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Meprobamate in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Meprobamate in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Meprobamate in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Meprobamate in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
There is limited information regarding Monitoring of Meprobamate in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Meprobamate in the drug label.
Overdosage
- Suicidal attempts with meprobamate have resulted in drowsiness, lethargy, stupor, ataxia, coma, shock, vasomotor, and respiratory collapse. Some suicidal attempts have been fatal.
- The following data on meprobamate tablets have been reported in the literature and from other sources. These data are not expected to correlate with each case (considering factors such as individual susceptibility and length of time from ingestion to treatment), but represent the usual ranges reported.
Acute simple overdose (meprobamate alone): Death has been reported with ingestion of as little as 12 g meprobamate and survival with as much as 40 g.
Blood Levels
- 0.5-2 mg% represents the usual blood level range of meprobamate after therapeutic doses. The level may occasionally be as high as 3 mg%.
- 3-10 mg% usually corresponds to findings of mild to moderate symptoms of overdosage, such as stupor or light coma.
- 10-20 mg% usually corresponds to deeper coma, requiring more intensive treatment. Some fatalities occur.
- At levels greater than 20 mg%, more fatalities than survivals can be expected.
Acute combined overdose (meprobamate with alcohol or other CNS depressants or psychotropic drugs): Since effects can be additive, a history of ingestion of a low dose of meprobamate plus any of these compounds (or of a relative low blood or tissue level) cannot be used as a prognostic indicator.
- In cases where excessive doses have been taken, sleep ensues rapidly and blood pressure, pulse, and respiratory rates are reduced to basal levels. Any drug remaining in the stomach should be removed and symptomatic therapy given. Should respiration or blood pressure become compromised, respiratory assistance, central nervous system stimulants, and pressor agents should be administered cautiously as indicated. Meprobamate is metabolized in the liver and excreted by the kidney. Diuresis, osmotic (mannitol) diuresis, peritoneal dialysis, and hemodialysis have been used successfully. Careful monitoring of urinary output is necessary and caution should be taken to avoid overhydration. Relapse and death, after initial recovery, have been attributed to incomplete gastric emptying and delayed absorption. Meprobamate can be measured in biological fluids by two methods: colorimetric (Hoffman, A.J. and Ludwig, B.J.: J Amer Pharm Assn 48: 740, 1959) and gas chromatographic (Douglas, J.F. et al: Anal Chem 39: 956, 1967).
Pharmacology
| |
| |
Meprobamate
| |
| Systematic (IUPAC) name | |
| [2-(carbamoyloxymethyl)-2-methyl-pentyl] carbamate | |
| Identifiers | |
| CAS number | |
| ATC code | N05 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 218.250 g/mol |
| SMILES | & |
| Physical data | |
| Density | 1.229 g/cm³ |
| Melt. point | 105–106 °C (221–223 °F) |
| Boiling point | 200 °C (392 °F) to 210 °C (410 °F) |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | Hepatic |
| Half life | 10 hours |
| Excretion | Renal |
| Therapeutic considerations | |
| Pregnancy cat. | |
| Legal status | |
| Routes | Oral |
Mechanism of Action
There is limited information regarding Meprobamate Mechanism of Action in the drug label.
Structure
Meprobamate is a white powder with a characteristic odor and a bitter taste. It is slightly soluble in water, freely soluble in acetone and alcohol, and sparingly soluble in ether. The structural formula of meprobamate is:

Meprobamate Tablets USP 200 mg and 400 mg for oral administration contain the following inactive ingredients: colloidal silicon dioxide, magnesium stearate, microcrystalline cellulose, sodium starch glycolate and pregelatinised starch.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Meprobamate in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Meprobamate in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Meprobamate in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Meprobamate in the drug label.
How Supplied
- Meprobamate Tablets USP 200 mg are white to off white, round, biconvex, uncoated tablets debossed with “L125” on one side and break line on other side.
- NDC 23155-128-03 Bottle of 30
- NDC 23155-128-01 Bottle of 100
- NDC 23155-128-10 Bottle of 1000
- Meprobamate Tablets USP 400 mg are white to off white, round, biconvex, uncoated tablets debossed with “L105” on one side and break line on other side.
- NDC 23155-129-03 Bottle of 30
- NDC 23155-129-01 Bottle of 100
- NDC 23155-129-10 Bottle of 1000
- Dispense in well-closed container with child-resistant closure.
Storage
- Store at controlled room temperature, excursions permitted to 15°C-30°C (59°F-86°F).
- Preserve in well closed container.
Images
Drug Images
{{#ask: Page Name::Meprobamate |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Meprobamate |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Meprobamate in the drug label.
Precautions with Alcohol
- Alcohol-Meprobamate interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
MEPROBAMATE
Look-Alike Drug Names
There is limited information regarding Meprobamate Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=Meprobamate
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Meprobamate |Label Name=Meprobamate ingredients and appearance.png
}}
{{#subobject:
|Label Page=Meprobamate |Label Name=Meprobamate fig01.jpg
}}
{{#subobject:
|Label Page=Meprobamate |Label Name=Meprobamate fig.jpg
}}