Membranous glomerulonephritis pathophysiology: Difference between revisions
No edit summary |
Sunny Kumar (talk | contribs) No edit summary |
||
| (53 intermediate revisions by 5 users not shown) | |||
| Line 1: | Line 1: | ||
__NOTOC__ | __NOTOC__ | ||
{{Membranous glomerulonephritis}} | {{Membranous glomerulonephritis}} | ||
{{CMG}}; {{AE}} {{SAH}} {{Pervaiz Laghari}} | |||
==Overview== | ==Overview== | ||
Membranous glomerulonephritis is caused by immune complex formation in the glomerulus. The immune complexes are formed by binding of antibodies to antigens in the [[glomerular basement membrane]]. The antigens damage the basement membrane and activates the immune response. The immune complex serves as an activator that triggers a response from the complement system. .PLA2R antigen detected within immune deposits by [[immunofluorescence]] of the [[biopsy]] specimen. Formation of the [[Immune complexes|immune complex]]. Immune complex formation results in release of cytokines which release membrane attack complex C5-C9. Release of C5-C9 lead to injury of podocyte which causes loss of glomerular permeablity. The damage to podocyte reults in proteinuria. | |||
==Pathophysiology== | ==Pathophysiology== | ||
* The membranous [[glomerulonephritis]] is a result of multiple changes | *The membranous [[glomerulonephritis]] is a result of multiple changes, which are:<ref name="pmid16159900">{{cite journal |vauthors=Cybulsky AV, Quigg RJ, Salant DJ |title=Experimental membranous nephropathy redux |journal=Am. J. Physiol. Renal Physiol. |volume=289 |issue=4 |pages=F660–71 |date=October 2005 |pmid=16159900 |pmc=1325222 |doi=10.1152/ajprenal.00437.2004 |url=}}</ref><ref name="pmid15800119">{{cite journal |vauthors=Nangaku M, Shankland SJ, Couser WG |title=Cellular response to injury in membranous nephropathy |journal=J. Am. Soc. Nephrol. |volume=16 |issue=5 |pages=1195–204 |date=May 2005 |pmid=15800119 |doi=10.1681/ASN.2004121098 |url=}}</ref><ref name="pmid15800113">{{cite journal |vauthors=Cunningham PN, Quigg RJ |title=Contrasting roles of complement activation and its regulation in membranous nephropathy |journal=J. Am. Soc. Nephrol. |volume=16 |issue=5 |pages=1214–22 |date=May 2005 |pmid=15800113 |doi=10.1681/ASN.2005010096 |url=}}</ref><ref name="pmid23364522">{{cite journal |vauthors=Kanigicherla D, Gummadova J, McKenzie EA, Roberts SA, Harris S, Nikam M, Poulton K, McWilliam L, Short CD, Venning M, Brenchley PE |title=Anti-PLA2R antibodies measured by ELISA predict long-term outcome in a prevalent population of patients with idiopathic membranous nephropathy |journal=Kidney Int. |volume=83 |issue=5 |pages=940–8 |date=May 2013 |pmid=23364522 |doi=10.1038/ki.2012.486 |url=}}</ref><ref name="pmid21323563">{{cite journal |vauthors=Debiec H, Ronco P |title=PLA2R autoantibodies and PLA2R glomerular deposits in membranous nephropathy |journal=N. Engl. J. Med. |volume=364 |issue=7 |pages=689–90 |date=February 2011 |pmid=21323563 |doi=10.1056/NEJMc1011678 |url=}}</ref><ref name="pmid22673885">{{cite journal |vauthors=Hoxha E, Kneißler U, Stege G, Zahner G, Thiele I, Panzer U, Harendza S, Helmchen UM, Stahl RA |title=Enhanced expression of the M-type phospholipase A2 receptor in glomeruli correlates with serum receptor antibodies in primary membranous nephropathy |journal=Kidney Int. |volume=82 |issue=7 |pages=797–804 |date=October 2012 |pmid=22673885 |doi=10.1038/ki.2012.209 |url=}}</ref><ref name="pmid23223223">{{cite journal |vauthors=Svobodova B, Honsova E, Ronco P, Tesar V, Debiec H |title=Kidney biopsy is a sensitive tool for retrospective diagnosis of PLA2R-related membranous nephropathy |journal=Nephrol. Dial. Transplant. |volume=28 |issue=7 |pages=1839–44 |date=July 2013 |pmid=23223223 |doi=10.1093/ndt/gfs439 |url=}}</ref> | ||
* | ** Membranous glomerulonephritis is caused by immune complex formation in the glomerulus. | ||
* The immune complex serves as an activator that triggers a response from the complement system | ** The immune complexes are formed by binding of antibodies to antigens in the [[glomerular basement membrane]]. | ||
** The antigens damage the basement membrane and activates the immune response. | |||
** The immune complex serves as an activator that triggers a response from the complement system. | |||
'''Phospholipase A2 receptor''' | '''Phospholipase A2 receptor''' | ||
* The [[M-type]] PLA2R is the major antigen in human idiopathic | * The [[M-type]] PLA2R is the major antigen in human idiopathic membranous glomerulonephritis. It is expressed in [[glomerular podocytes|glomerular podocytes.]]T | ||
* | * here was no colocalization of PLA2R in secondary [[membranous glomerulonephritis]] [[biopsies]] | ||
* PLA2R antigen detected within immune deposits by immunofluorescence of the biopsy specimen. | * .PLA2R antigen detected within immune deposits by [[immunofluorescence]] of the [[biopsy]] specimen. Formation of the [[Immune complexes|immune complex]]. | ||
* Immune complex formation results in release of cytokines which release membrane attack complex C5-C9. | |||
* Release of C5-C9 lead to injury of podocyte which causes loss of glomerular permeablity. | |||
* | * The damage to podocyte reults in proteinuria. | ||
* | |||
* The | |||
{{Family tree/start}} | {{Family tree/start}} | ||
{{Family tree | | | | A01 | | | | A02 | |A01=HLA susceptibility 1|A02=Environmental factors}} | {{Family tree | | | | A01 | | | | A02 | |A01=HLA susceptibility 1|A02=Environmental factors}} | ||
{{Family tree | | | | |`|-|-|v|-|-|'| | | |}} | {{Family tree | | | | |`|-|-|v|-|-|'| | | |}} | ||
{{Family tree | | | | | | |B01| | | | | |B01=Variant of PLA2R1 on podocyte surface}} | {{Family tree | | | | | | | B01 | | | | | |B01=Variant of PLA2R1 on podocyte surface}} | ||
{{Family tree | | | | | | |!| | | | | |}} | {{Family tree | | | | | | | |!| | | | | |}} | ||
{{Family tree | | | | | | |C01| | | | | |C01=Innate immunity activation and inflammation<br>dendritic cell sense epitope of PLA2R1<br>and present them for adaptive immunity}} | {{Family tree | | | | | | | C01 | | | | | |C01=Innate immunity activation and inflammation<br>dendritic cell sense epitope of PLA2R1<br>and present them for adaptive immunity}} | ||
{{Family tree | | | | | | |!| | | | | |}} | {{Family tree | | | | | | | |!| | | | | |}} | ||
{{Family tree | | | | | | |D01| | | | | |D01=Production of auto-immune antibody IgG4/IgG1 which attach them self to epitope on podocyte surface}} | {{Family tree | | | | | | | D01 | | | | | |D01=Production of auto-immune antibody IgG4/IgG1 which attach them self to epitope on podocyte surface}} | ||
{{Family tree | | | | | | |!| | | | | |}} | {{Family tree | | | | | | | |!| | | | | |}} | ||
{{Family tree | | | | | | |E01| | | | | |E01=In Situ formation and shedding of subepithelial immune complex}} | {{Family tree | | | | | | | E01 | | | | | |E01=In Situ formation and shedding of subepithelial immune complex}} | ||
{{Family tree | | {{Family tree | | | | | | | |!| | | | | |}} | ||
{{Family tree | | | | | | | J01 | | | | | |J01=Which lead to<br>cytokine release<br>oxygen derivative release<br>membrane attack complex C5-C9}} | |||
{{Family tree | | | | | | | |!| | | | | |}} | |||
{{Family tree | | | | | | |J01| | | | | |J01=Which lead to<br>cytokine release<br>oxygen derivative release<br>membrane attack complex C5-C9}} | {{Family tree | | | | | | | H01 | | | | | |H01=Podocyte injury by apoptosis<br>altered lectin cytoskeleton<br>loss of silt pore integrity<br>loss of glomerular permeability <br>proteinuria}} | ||
{{Family tree | | | | | | |!| | | | | |}} | |||
{{Family tree | | | | | | |H01| | | | | |H01=Podocyte injury by apoptosis<br>altered lectin cytoskeleton<br>loss of silt pore integrity<br>loss of glomerular permeability <br>proteinuria}} | |||
{{familytree/end}} | {{familytree/end}} | ||
==Genetics== | ==Genetics== | ||
* Single-nucleotide polymorphisms (SNPs) at two loci that are highly associated with idiopathic | * Single-nucleotide polymorphisms (SNPs) at two loci that are highly associated with idiopathic membranous glomerulonephritis.<ref name="pmid21323563">{{cite journal |vauthors=Debiec H, Ronco P |title=PLA2R autoantibodies and PLA2R glomerular deposits in membranous nephropathy |journal=N. Engl. J. Med. |volume=364 |issue=7 |pages=689–90 |date=February 2011 |pmid=21323563 |doi=10.1056/NEJMc1011678 |url=}}</ref><ref name="pmid22673885">{{cite journal |vauthors=Hoxha E, Kneißler U, Stege G, Zahner G, Thiele I, Panzer U, Harendza S, Helmchen UM, Stahl RA |title=Enhanced expression of the M-type phospholipase A2 receptor in glomeruli correlates with serum receptor antibodies in primary membranous nephropathy |journal=Kidney Int. |volume=82 |issue=7 |pages=797–804 |date=October 2012 |pmid=22673885 |doi=10.1038/ki.2012.209 |url=}}</ref> | ||
* The two loci are within the genes for the PLA2R on chromosome 2q24 | * The two loci are within the genes for the [[PLA2R]] on [[chromosome]] 2q24. | ||
* | * The human leukocyte antigen (HLA) complex class II alpha chain 1A [[(''HLA-DQA1'')]] on chromosome 6p21. | ||
* The [[PLA2R]] has been identified as a major [[antigen]] in idiopathic membranous glomerulonephritis. | |||
==Associated Conditions== | ==Associated Conditions== | ||
Consitions associated with | Consitions associated with membranous glomerulonephritis include:<ref name="pmid10495797">{{cite journal |vauthors=Wasserstein AG |title=Membranous glomerulonephritis |journal=J. Am. Soc. Nephrol. |volume=8 |issue=4 |pages=664–74 |date=April 1997 |pmid=10495797 |doi= |url=}}</ref> | ||
*[[Hepatitis B/History & Symptoms|Hepatitis B]] | *[[Hepatitis B/History & Symptoms|Hepatitis B]] | ||
*[[Hepatitis C]] | *[[Hepatitis C]] | ||
| Line 52: | Line 48: | ||
*[[SLE|Systemic Lupus Erythematosis]] | *[[SLE|Systemic Lupus Erythematosis]] | ||
*Malignancy | *Malignancy | ||
**Lung | **[[Lung]] | ||
**Breast | **[[Breast]] | ||
**Colon | **[[Colon]] | ||
**Stomach | **[[Stomach]] | ||
**Kidney | **[[Kidney]] | ||
**Leukemia | **[[Leukemia]] | ||
**Lymphomas ( | **[[Lymphomas]] ([[Hodgkin]]’s and [[non-Hodgkin]]’s) | ||
== Gross Pathology == | == Gross Pathology == | ||
* | * On gross pathology examination there is no characteristic findings present | ||
==Microscopic Pathology== | ==Microscopic Pathology== | ||
Microscopic pathologic findings characteristic of | Microscopic pathologic findings characteristic of membranous glomerulonephritis include:<ref name="pmid16159900">{{cite journal |vauthors=Cybulsky AV, Quigg RJ, Salant DJ |title=Experimental membranous nephropathy redux |journal=Am. J. Physiol. Renal Physiol. |volume=289 |issue=4 |pages=F660–71 |date=October 2005 |pmid=16159900 |pmc=1325222 |doi=10.1152/ajprenal.00437.2004 |url=}}</ref><ref name="pmid15800119">{{cite journal |vauthors=Nangaku M, Shankland SJ, Couser WG |title=Cellular response to injury in membranous nephropathy |journal=J. Am. Soc. Nephrol. |volume=16 |issue=5 |pages=1195–204 |date=May 2005 |pmid=15800119 |doi=10.1681/ASN.2004121098 |url=}}</ref> | ||
* Early biopsies may be normal | * Early biopsies may be normal. | ||
* Later: uniform diffuse capillary wall thickening without hypercellularity, | * Later: uniform diffuse capillary wall thickening without hypercellularity, no [[mesangial]] [[sclerosis]] and [[inflammatory]] cells. | ||
* Proximal convoluted tubules: hyaline droplets, reflecting protein reabsorption | * Proximal convoluted tubules: [[hyaline]] droplets, reflecting protein reabsorption. | ||
* Membrane thickening and narrow capillary lumina. | * Membrane thickening and narrow capillary lumina. | ||
* Mesangial sclerosis and glomerulosclerosis | * Mesangial [[sclerosis]] and [[glomerulosclerosis]]. | ||
'''Immunofluorescence''' | '''Immunofluorescence''' | ||
* Granular diffuse peripheral deposits, usually IgG and C3, also C5b-C9 and occasionally IgM or IgA | * Granular diffuse peripheral deposits, usually [[IgG]] and [[C3]], also [[C5b]]-[[C9]] and occasionally [[IgM]] or [[IgA]]. | ||
* C4d immunostaining may be diagnostic. | * [[C4d]] immunostaining may be diagnostic. | ||
The microscopic,immunofluorscence and electron microscopic features are listed in the following table:<ref name="pmid16159900">{{cite journal |vauthors=Cybulsky AV, Quigg RJ, Salant DJ |title=Experimental membranous nephropathy redux |journal=Am. J. Physiol. Renal Physiol. |volume=289 |issue=4 |pages=F660–71 |date=October 2005 |pmid=16159900 |pmc=1325222 |doi=10.1152/ajprenal.00437.2004 |url=}}</ref><ref name="pmid15800119">{{cite journal |vauthors=Nangaku M, Shankland SJ, Couser WG |title=Cellular response to injury in membranous nephropathy |journal=J. Am. Soc. Nephrol. |volume=16 |issue=5 |pages=1195–204 |date=May 2005 |pmid=15800119 |doi=10.1681/ASN.2004121098 |url=}}</ref> | |||
{| class="wikitable" | {| class="wikitable" | ||
! | ! style="background:#4479BA; color: #FFFFFF;" align="center" + |Stage | ||
! | ! style="background:#4479BA; color: #FFFFFF;" align="center" + |Glomerular Basement Membrane | ||
! | ! style="background:#4479BA; color: #FFFFFF;" align="center" + |Immunofluorescence | ||
! | ! style="background:#4479BA; color: #FFFFFF;" align="center" + |Electron Microscopy | ||
|- | |- | ||
| | | style="background:#DCDCDC;" align="center" + |Stage 1 | ||
| | | style="background:#F5F5F5;" align="center" + |Normal or slightly thickned BM | ||
| | | style="background:#F5F5F5;" align="center" + |Fine granular IgG, C3 | ||
|subepithelial electron dense deposits | | style="background:#F5F5F5;" align="center" + |Scattered small subepithelial electron dense deposits no foot effacement | ||
|- | |- | ||
| | | style="background:#DCDCDC;" align="center" + |Stage 2 | ||
| | | style="background:#F5F5F5;" align="center" + |Moderately thickened BM with spikes and vacuolization | ||
| | | style="background:#F5F5F5;" align="center" + |Granular IgG, C3 | ||
| | | style="background:#F5F5F5;" align="center" + |Diffuse spikes due to subepithelial deposits, diffuse foot process effacement | ||
|- | |- | ||
| | | style="background:#DCDCDC;" align="center" + |Stage 3 | ||
| | | style="background:#F5F5F5;" align="center" + |Moderately thickened BM residual spikes and vacuoles | ||
| | | style="background:#F5F5F5;" align="center" + |Chain like appearance IF, coarsely granular IgG, C3 | ||
| | | style="background:#F5F5F5;" align="center" + |Intramembraneous deposits, spikes, neomembrane formation and diffuse foot process effacement | ||
|- | |||
| style="background:#DCDCDC;" align="center" + |Stage 4 | |||
| style="background:#F5F5F5;" align="center" + |Markedly thick GBM, few spikes, vacoules and glomerulosclerosis | |||
| style="background:#F5F5F5;" align="center" + |Focal IgG, C3 | |||
| style="background:#F5F5F5;" align="center" + |Sclerotic GBM, few deposits and lacunae | |||
|} | |} | ||
<div align="center"> | |||
<div align="center"> | |||
<gallery heights="175" widths="125"> | <gallery heights="175" widths="125"> | ||
image:Membranous GN.jpg|Membranous Glomerulonephritis: Electron micrography. An excellent example to show thickened basement membrane and immune complexes. | image:Membranous GN.jpg|Membranous Glomerulonephritis: Electron micrography. An excellent example to show thickened basement membrane and immune complexes. | ||
| Line 121: | Line 108: | ||
</div> | </div> | ||
==References== | |||
{{Reflist|2}} | |||
{{WH}} | |||
{{WS}} | |||
==References== | ==References== | ||
{{reflist|2}} | {{reflist|2}} | ||
{{WH}} | {{WH}} | ||
{{WS}} | {{WS}} | ||
[[Category:(name of the system)]] | |||
[[Category: | [[Category:NephrologY]] | ||
[[Category: | [[Category:Up-to-date]] | ||
[[Category: | |||
Latest revision as of 09:39, 17 October 2020
|
Membranous glomerulonephritis Microchapters |
|
Differentiating Membranous glomerulonephritis from other Diseases |
|---|
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
Membranous glomerulonephritis pathophysiology On the Web |
|
American Roentgen Ray Society Images of Membranous glomerulonephritis pathophysiology |
|
Directions to Hospitals Treating Membranous glomerulonephritis |
|
Risk calculators and risk factors for Membranous glomerulonephritis pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Syed Ahsan Hussain, M.D.[2] Pervaiz Laghari, MD[3]
Overview
Membranous glomerulonephritis is caused by immune complex formation in the glomerulus. The immune complexes are formed by binding of antibodies to antigens in the glomerular basement membrane. The antigens damage the basement membrane and activates the immune response. The immune complex serves as an activator that triggers a response from the complement system. .PLA2R antigen detected within immune deposits by immunofluorescence of the biopsy specimen. Formation of the immune complex. Immune complex formation results in release of cytokines which release membrane attack complex C5-C9. Release of C5-C9 lead to injury of podocyte which causes loss of glomerular permeablity. The damage to podocyte reults in proteinuria.
Pathophysiology
- The membranous glomerulonephritis is a result of multiple changes, which are:[1][2][3][4][5][6][7]
- Membranous glomerulonephritis is caused by immune complex formation in the glomerulus.
- The immune complexes are formed by binding of antibodies to antigens in the glomerular basement membrane.
- The antigens damage the basement membrane and activates the immune response.
- The immune complex serves as an activator that triggers a response from the complement system.
Phospholipase A2 receptor
- The M-type PLA2R is the major antigen in human idiopathic membranous glomerulonephritis. It is expressed in glomerular podocytes.T
- here was no colocalization of PLA2R in secondary membranous glomerulonephritis biopsies
- .PLA2R antigen detected within immune deposits by immunofluorescence of the biopsy specimen. Formation of the immune complex.
- Immune complex formation results in release of cytokines which release membrane attack complex C5-C9.
- Release of C5-C9 lead to injury of podocyte which causes loss of glomerular permeablity.
- The damage to podocyte reults in proteinuria.
| HLA susceptibility 1 | Environmental factors | ||||||||||||||||||||||||||||
| Variant of PLA2R1 on podocyte surface | |||||||||||||||||||||||||||||
| Innate immunity activation and inflammation dendritic cell sense epitope of PLA2R1 and present them for adaptive immunity | |||||||||||||||||||||||||||||
| Production of auto-immune antibody IgG4/IgG1 which attach them self to epitope on podocyte surface | |||||||||||||||||||||||||||||
| In Situ formation and shedding of subepithelial immune complex | |||||||||||||||||||||||||||||
| Which lead to cytokine release oxygen derivative release membrane attack complex C5-C9 | |||||||||||||||||||||||||||||
| Podocyte injury by apoptosis altered lectin cytoskeleton loss of silt pore integrity loss of glomerular permeability proteinuria | |||||||||||||||||||||||||||||
Genetics
- Single-nucleotide polymorphisms (SNPs) at two loci that are highly associated with idiopathic membranous glomerulonephritis.[5][6]
- The two loci are within the genes for the PLA2R on chromosome 2q24.
- The human leukocyte antigen (HLA) complex class II alpha chain 1A (''HLA-DQA1'') on chromosome 6p21.
- The PLA2R has been identified as a major antigen in idiopathic membranous glomerulonephritis.
Associated Conditions
Consitions associated with membranous glomerulonephritis include:[8]
- Hepatitis B
- Hepatitis C
- Congenital Syphilis
- Systemic Lupus Erythematosis
- Malignancy
Gross Pathology
- On gross pathology examination there is no characteristic findings present
Microscopic Pathology
Microscopic pathologic findings characteristic of membranous glomerulonephritis include:[1][2]
- Early biopsies may be normal.
- Later: uniform diffuse capillary wall thickening without hypercellularity, no mesangial sclerosis and inflammatory cells.
- Proximal convoluted tubules: hyaline droplets, reflecting protein reabsorption.
- Membrane thickening and narrow capillary lumina.
- Mesangial sclerosis and glomerulosclerosis.
Immunofluorescence
- Granular diffuse peripheral deposits, usually IgG and C3, also C5b-C9 and occasionally IgM or IgA.
- C4d immunostaining may be diagnostic.
The microscopic,immunofluorscence and electron microscopic features are listed in the following table:[1][2]
| Stage | Glomerular Basement Membrane | Immunofluorescence | Electron Microscopy |
|---|---|---|---|
| Stage 1 | Normal or slightly thickned BM | Fine granular IgG, C3 | Scattered small subepithelial electron dense deposits no foot effacement |
| Stage 2 | Moderately thickened BM with spikes and vacuolization | Granular IgG, C3 | Diffuse spikes due to subepithelial deposits, diffuse foot process effacement |
| Stage 3 | Moderately thickened BM residual spikes and vacuoles | Chain like appearance IF, coarsely granular IgG, C3 | Intramembraneous deposits, spikes, neomembrane formation and diffuse foot process effacement |
| Stage 4 | Markedly thick GBM, few spikes, vacoules and glomerulosclerosis | Focal IgG, C3 | Sclerotic GBM, few deposits and lacunae |
-
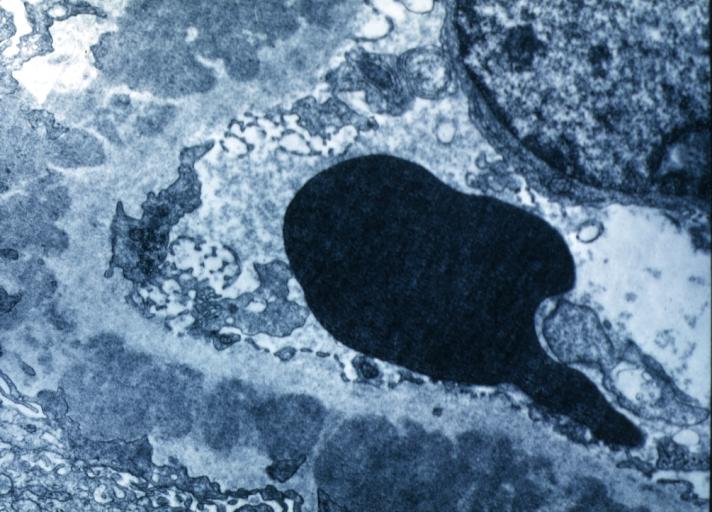
Membranous Glomerulonephritis: Electron micrography. An excellent example to show thickened basement membrane and immune complexes.
-
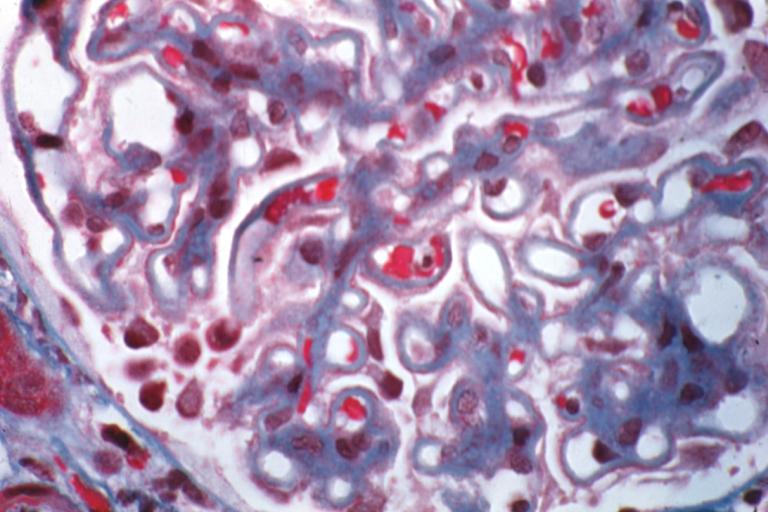
Membranous Glomerulonephritis: Micro trichrome high mag excellent to show thickened capillary basement membranes
-
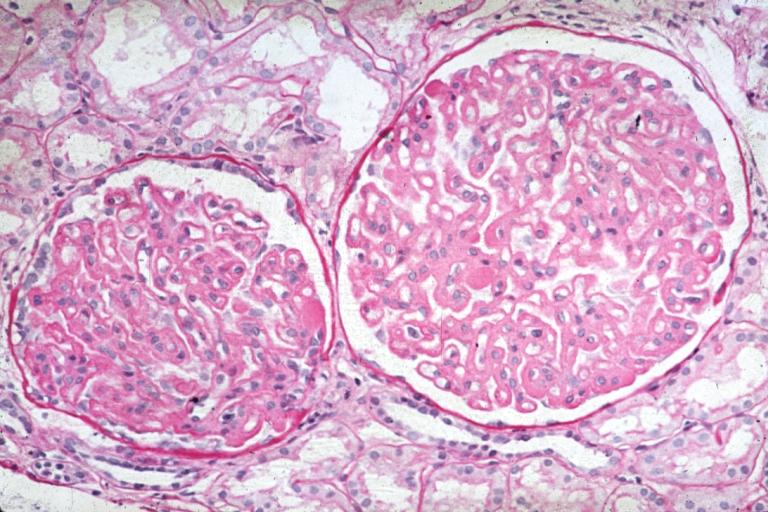
Membranous Glomerulonephritis: Micro PAS high mag excellent example of this lesion
-
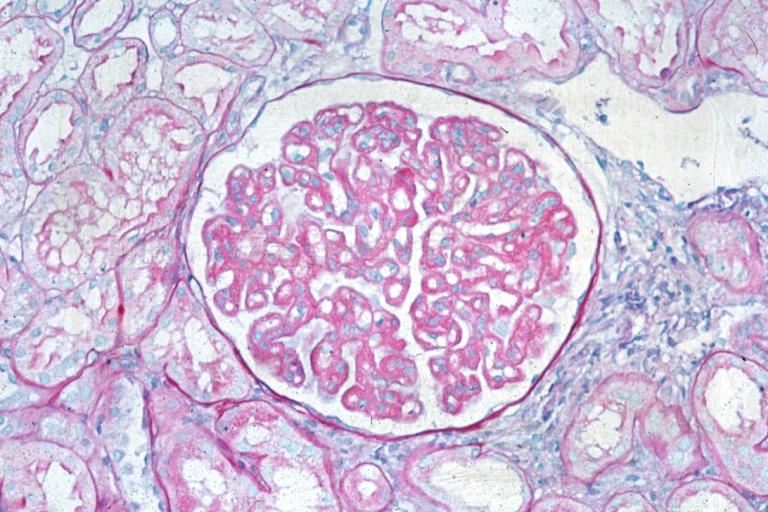
Membranous Glomerulonephritis: Micro PAS med mag
References
- ↑ 1.0 1.1 1.2 Cybulsky AV, Quigg RJ, Salant DJ (October 2005). "Experimental membranous nephropathy redux". Am. J. Physiol. Renal Physiol. 289 (4): F660–71. doi:10.1152/ajprenal.00437.2004. PMC 1325222. PMID 16159900.
- ↑ 2.0 2.1 2.2 Nangaku M, Shankland SJ, Couser WG (May 2005). "Cellular response to injury in membranous nephropathy". J. Am. Soc. Nephrol. 16 (5): 1195–204. doi:10.1681/ASN.2004121098. PMID 15800119.
- ↑ Cunningham PN, Quigg RJ (May 2005). "Contrasting roles of complement activation and its regulation in membranous nephropathy". J. Am. Soc. Nephrol. 16 (5): 1214–22. doi:10.1681/ASN.2005010096. PMID 15800113.
- ↑ Kanigicherla D, Gummadova J, McKenzie EA, Roberts SA, Harris S, Nikam M, Poulton K, McWilliam L, Short CD, Venning M, Brenchley PE (May 2013). "Anti-PLA2R antibodies measured by ELISA predict long-term outcome in a prevalent population of patients with idiopathic membranous nephropathy". Kidney Int. 83 (5): 940–8. doi:10.1038/ki.2012.486. PMID 23364522.
- ↑ 5.0 5.1 Debiec H, Ronco P (February 2011). "PLA2R autoantibodies and PLA2R glomerular deposits in membranous nephropathy". N. Engl. J. Med. 364 (7): 689–90. doi:10.1056/NEJMc1011678. PMID 21323563.
- ↑ 6.0 6.1 Hoxha E, Kneißler U, Stege G, Zahner G, Thiele I, Panzer U, Harendza S, Helmchen UM, Stahl RA (October 2012). "Enhanced expression of the M-type phospholipase A2 receptor in glomeruli correlates with serum receptor antibodies in primary membranous nephropathy". Kidney Int. 82 (7): 797–804. doi:10.1038/ki.2012.209. PMID 22673885.
- ↑ Svobodova B, Honsova E, Ronco P, Tesar V, Debiec H (July 2013). "Kidney biopsy is a sensitive tool for retrospective diagnosis of PLA2R-related membranous nephropathy". Nephrol. Dial. Transplant. 28 (7): 1839–44. doi:10.1093/ndt/gfs439. PMID 23223223.
- ↑ Wasserstein AG (April 1997). "Membranous glomerulonephritis". J. Am. Soc. Nephrol. 8 (4): 664–74. PMID 10495797.