Lipoic acid: Difference between revisions
m (Robot: Automated text replacement (-{{SIB}} + & -{{EH}} + & -{{EJ}} + & -{{Editor Help}} + & -{{Editor Join}} +)) |
No edit summary |
||
| (3 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{Chembox | |||
{{Chembox | |||
| Watchedfields = changed | |||
| verifiedrevid = 477002603 | |||
| ImageFile_Ref = {{chemboximage|correct|??}} | |||
| ImageFile = Lipoic-acid-2D-skeletal.png | | ImageFile = Lipoic-acid-2D-skeletal.png | ||
| ImageFile1 = Lipoic-acid-3D-vdW.png | | ImageFile1 = Lipoic-acid-3D-vdW.png | ||
| IUPACName = | | ImageFile2 = Lipoic acid ball and stick.png | ||
| OtherNames = α- | | IUPACName = (''R'')-5-(1,2-dithiolan-3-yl)pentanoic acid | ||
| Section1 = {{Chembox Identifiers | | OtherNames = α-Lipoic acid (alpha lipoic acid); Thioctic acid; 6,8-Dithiooctanoic acid | ||
| | |Section1={{Chembox Identifiers | ||
| | | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| | | ChemSpiderID = 5886 | ||
| | | KEGG_Ref = {{keggcite|correct|kegg}} | ||
| KEGG = C16241 | |||
| Section2 = {{Chembox Properties | | InChI = 1/C8H14O2S2/c9-8(10)4-2-1-3-7-5-6-11-12-7/h7H,1-6H2,(H,9,10)/t7-/m1/s1 | ||
| | | InChIKey = AGBQKNBQESQNJD-SSDOTTSWBZ | ||
| | | ChEMBL_Ref = {{ebicite|correct|EBI}} | ||
| | | ChEMBL = 134342 | ||
| | | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | ||
| | | StdInChI = 1S/C8H14O2S2/c9-8(10)4-2-1-3-7-5-6-11-12-7/h7H,1-6H2,(H,9,10)/t7-/m1/s1 | ||
| | | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | ||
| StdInChIKey = AGBQKNBQESQNJD-SSDOTTSWSA-N | |||
| Section3 = {{Chembox Hazards | | CASNo = 1200-22-2 | ||
| | | CASNo_Comment = (''R'') | ||
| | | CASNo1 = 1077-28-7 | ||
| | | CASNo1_Comment = (racemate) | ||
| | | CASNo_Ref = {{cascite|correct|CAS}} | ||
| PubChem = 6112 | |||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| DrugBank = DB00166 | |||
| ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| ChEBI = 30314 | |||
| SMILES = O=C(O)CCCC[C@H]1SSCC1 | |||
| MeSHName = Lipoic+acid | |||
| ATCCode_prefix = A16 | |||
| ATCCode_suffix = AX01 | |||
}} | |||
|Section2={{Chembox Properties | |||
| C=8|H=14|O=2|S=2 | |||
| Appearance = Yellow needle-like crystals | |||
| Solubility = Soluble as sodium salt | |||
| SolubleOther = Soluble | |||
| Solvent = ethanol | |||
| Density = | |||
| MeltingPt = | |||
| BoilingPt = | |||
}} | |||
|Section3={{Chembox Hazards | |||
| MainHazards = | |||
| FlashPt = | |||
| AutoignitionPt = | |||
}} | |||
|Section4={{Chembox Pharmacology | |||
| Bioavail = 30% (oral)<ref>{{cite journal |last1= Teichert |first1= J |last2= Hermann |first2= R |last3= Ruus |first3= P |last4= Preiss |first4= R |title= Plasma kinetics, metabolism, and urinary excretion of alpha-lipoic acid following oral administration in healthy volunteers |journal= [[The Journal of Clinical Pharmacology]] |volume= 43 |issue= 11 |pages= 1257–67 |date= November 2003 |pmid= 14551180 |doi= 10.1177/0091270003258654 }}</ref> | |||
}} | |||
|Section8={{Chembox Related | |||
| OtherCpds = [[Lipoamide]]<br>[[Asparagusic acid]] | |||
}} | |||
}} | }} | ||
__NOTOC__ | |||
{{SI}} | |||
{{CMG}} | {{CMG}} | ||
==Overview== | |||
'''Lipoic acid''' ('''LA'''), also known as '''α-lipoic acid''' and '''alpha lipoic acid''' ('''ALA''') and '''thioctic acid''' is an [[organosulfur compound]] derived from [[caprylic acid|octanoic acid]]. ALA is made in animals normally, and is essential for [[aerobic metabolism]]. It is also manufactured and is available as a [[dietary supplement]] in some countries where it is marketed as an [[antioxidant]], and is available as a [[pharmaceutical drug]] in other countries. | |||
==Physical and chemical properties== | |||
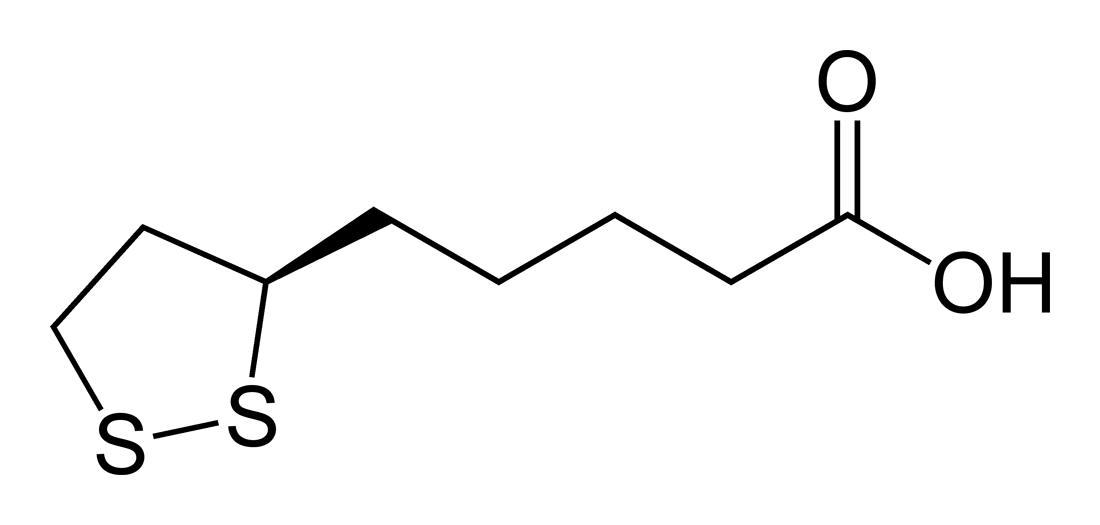
Lipoic acid (LA), also known as α-lipoic acid<ref name= "Shay08"/> and alpha lipoic acid (ALA) and thioctic acid<ref>{{cite journal |last1= Reljanovic |first1= M |last2= Reichel |first2= G |last3= Rett |first3= K |last4= Lobisch |first4= M |last5= Schuette |first5= K |last6= Möller |first6= W |last7= Tritschler |first7= HJ |last8= Mehnert |first8= H |displayauthors= 4 |title= Treatment of diabetic polyneuropathy with the antioxidant thioctic acid (alpha-lipoic acid): A two year multicenter randomized double-blind placebo-controlled trial (ALADIN II). Alpha Lipoic Acid in Diabetic Neuropathy |journal= [[Free Radical Research]] |volume= 31 |issue= 3 |pages= 171–9 |date= September 1999 |pmid= 10499773 |doi= 10.1080/10715769900300721}}</ref> is an [[organosulfur compound]] derived from [[caprylic acid|octanoic acid]]. LA contains two sulfur atoms (at C6 and C8) connected by a [[disulfide bond]] and is thus considered to be oxidized although either sulfur atom can exist in higher oxidation states. | |||
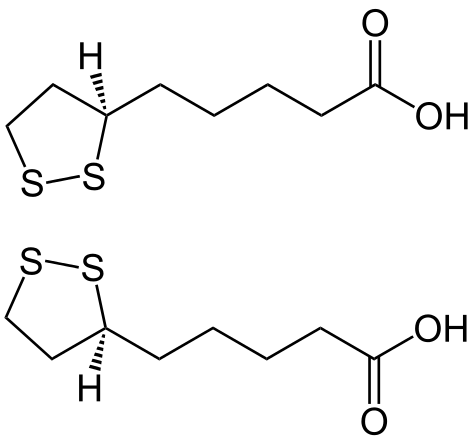
The carbon atom at C6 is [[chirality (chemistry)|chiral]] and the molecule exists as two [[enantiomers]] (''R'')-(+)-lipoic acid (RLA) and (''S'')-(-)-lipoic acid (SLA) and as a [[racemic mixture]] (''R''/''S'')-lipoic acid (R/S-LA). | |||
LA appears physically as a yellow solid and structurally contains a terminal carboxylic acid and a terminal dithiolane ring.{{citation needed|date=April 2014}} | |||
For use in [[dietary supplement]] materials and [[compounding]] pharmacies, the [[United States Pharmacopeia|USP]] has established an official monograph for R/S-LA.<ref>{{cite web |url= http://www.drugfuture.com/Pharmacopoeia/USP32/pub/data/v32270/usp32nf27s0_m45550.html |title= Alpha Lipoic Acid C<sub>8</sub>H<sub>1</sub><sub>4</sub>O<sub>2</sub>S<sub>2</sub> 206.33 |website= drugfuture.com |accessdate= 2014-07-05}} Reproduction of {{cite book |title= [[United States Pharmacopeia|USP32-NF27]] |page= 1042}} Also in {{cite journal |title= Pharmacopeial Forum |volume= 34 |issue= 5 |page= 1209}}</ref> | |||
==Biological function== | |||
"Lipoate" is the [[conjugate base]] of lipoic acid, and the most prevalent form of LA under physiologic conditions. Most endogenously produced RLA is not “free” because octanoic acid, the precursor to RLA, is bound to the enzyme complexes prior to enzymatic insertion of the sulfur atoms. As a cofactor, RLA is covalently attached by an amide bond to a terminal lysine residue of the enzyme’s lipoyl domains. One of the most studied roles of RLA is as a cofactor of the [[pyruvate dehydrogenase complex]] (PDC or PDHC), though it is a cofactor in other enzymatic systems as well (described below). | |||
Only the (''R'')-(+)-enantiomer (RLA) exists in nature and is essential for [[aerobic metabolism]] because RLA is an essential [[Cofactor (biochemistry)|cofactor]] of many enzyme complexes.<ref>{{cite journal |doi= 10.1016/S0168-1656(97)00135-1 |pmid= 9383983 |last1= Raddatz |first1= G |last2= Bisswanger |first2= H |title= Receptor site and stereospecifity of dihydrolipoamide dehydrogenase for R- and S-lipoamide: A molecular modeling study |journal= [[Journal of Biotechnology]] |volume= 58 |issue= 2 |date= 17 October 1997 |pages= 89–100}}</ref> | |||
===Biosynthesis and attachment=== | |||
The precursor to lipoic acid, [[octanoic acid]], is made via [[fatty acid biosynthesis]] in the form of octanoyl-[[acyl carrier protein]]. In [[eukaryotes]], a second fatty acid biosynthetic pathway in [[mitochondria]] is used for this purpose.<ref>{{cite journal |last1= Cronan |first1= JE |last2= Fearnley |first2= IM |last3= Walker |first3= JE |date= 29 August 2005 |title= Mammalian mitochondria contain a soluble acyl carrier protein |url= http://www.febsletters.org/article/S0014-5793%2805%2900945-2/fulltext |journal= [[FEBS Letters]] |volume= 579 |issue= 21 |pages= 4892–6|pmid= 16109413 |doi= 10.1016/j.febslet.2005.07.077 }}</ref><ref>{{cite journal |last1= Jordan |first1= SW |last2= Cronan |first2= JE, Jr. |year= 1997 |title= A new metabolic link. The acyl carrier protein of lipid synthesis donates lipoic acid to the pyruvate dehydrogenase complex in ''Escherichia coli'' and mitochondria |journal= [[Journal of Biological Chemistry]] |volume= 272 |issue= 29 |pages= 17903–6 |pmid= 9218413 |doi= 10.1074/jbc.272.29.17903 |url= http://www.jbc.org/content/272/29/17903.full}}</ref> The octanoate is transferred as a thioester of [[acyl carrier protein]] from fatty acid biosynthesis to an [[amide]] of the lipoyl domain protein by an [[enzyme]] called an octanoyltransferase. Two hydrogens of octanoate are replaced with sulfur groups via a [[radical SAM]] mechanism, by [[lipoyl synthase]] <ref>{{cite journal |last1= Cicchillo |first1= RM |last2= Booker |last5= Booker |first2= SJ |year= 2005 |title= Mechanistic investigations of lipoic acid biosynthesis in ''E. coli'': Both sulfur atoms in lipoic acid are contributed by the same lipoyl synthase polypeptide |journal= [[Journal of the American Chemical Society]] |volume= 127 |issue= 9 |pages= 2860–1 |pmid= 15740115 |doi= 10.1021/ja042428u }}</ref> As a result, lipoic acid is synthesized attached to proteins and no free lipoic acid is produced. Lipoic acid can be removed whenever proteins are degraded and by action of the enzyme lipoamidase.<ref>{{cite journal |last1= Jiang |first1= Y |last2= Cronan |first2= JE |year= 2005 |title= Expression cloning and demonstration of ''Enterococcus faecalis'' lipoamidase (pyruvate dehydrogenase inactivase) as a Ser-Ser-Lys triad amidohydrolase |journal= [[Journal of Biological Chemistry]] |volume= 280 |issue= 3 |pages= 2244–56 |pmid= 15528186 |doi= 10.1074/jbc.+M408612200 |doi_brokendate= 2015-01-14 }}</ref> Free lipoate can be used by some organisms an enzyme called lipoate protein ligase that attaches it covalently to the correct protein. The [[ligase]] activity of this [[enzyme]] requires [[Adenosine triphosphate|ATP]].<ref>{{cite journal |last1= Cronan |first1= JE |title= Function, attachment and synthesis of lipoic acid in Escherichia coli |journal= Advances in microbial physiology |last2= Zhao |first2= X |last3= Jiang |first3= Y |year= 2005 |chapter= Function, Attachment and Synthesis of Lipoic Acid in ''Escherichia coli'' |series= Advances in Microbial Physiology |volume= 50 |pages= 103–46 |pmid= 16221579 |doi= 10.1016/S0065-2911(05)50003-1 |isbn= 9780120277506 |editor-first= RK |editor-last= Poole}} | |||
</ref> | |||
===Enzymatic Activity=== | |||
Lipoic acid is [[Cofactor (biochemistry)|cofactor]] for at least five [[enzyme]] systems. Two of these are in the [[citric acid cycle]] through which many organisms turn nutrients into energy. Lipoylated [[enzymes]] have lipoic acid attached to them covalently. The lipoyl group transfers [[acyl]] groups in 2-oxoacid dehydrogenase complexes, and [[methylamine]] group in the [[glycine cleavage complex]] or [[glycine dehydrogenase]]. | |||
2-Oxoacid dehydrogenase transfer reactions occur by a similar mechanism in: | |||
# the [[pyruvate dehydrogenase complex]] | |||
# the α-ketoglutarate dehydrogenase or [[2-oxoglutarate dehydrogenase]] complex | |||
# the [[branched chain oxoacid dehydrogenase|branched-chain oxoacid dehydrogenase]] (BCDH) complex | |||
# the [[acetoin dehydrogenase]] complex. | |||
The most-studied of these is the pyruvate dehydrogenase complex. These complexes have three central subunits: E1-3, which are the decarboxylase, lipoyl transferase, and [[dihydrolipoamide dehydrogenase]], respectively. These complexes have a central E2 core and the other subunits surround this core to form the complex. In the gap between these two subunits, the lipoyl domain ferries intermediates between the active sites.<ref> | |||
{{cite journal |last1= Milne |first1= JL |last2= Wu |first2= X |last3= Borgnia |first3= MJ |last4= Lengyel |first4= JS |last5= Brooks |first5= BR |last6= Shi |first6= D |last7= Perham |first7= RN |last8= Subramaniam |first8= S |displayauthors= 4 | title= Molecular structure of a 9-MDa icosahedral pyruvate dehydrogenase subcomplex containing the E2 and E3 enzymes using cryoelectron microscopy | journal= [[Journal of Biological Chemistry]] |year= 2006 |pages= 4364–70 |volume= 281 |issue= 7 |doi= 10.1074/jbc.M504363200 |pmid= 16308322 |pmc= 1647297}}</ref><ref>{{cite journal |last1= Murphy |first1=GE |last2= Jensen |first2= GJ | title= Electron cryotomography of the E. coli pyruvate and 2-oxoglutarate dehydrogenase complexes | journal= [[Structure (journal)|Structure]] |year= 2005 |pages= 1765–73 |volume= 13 |issue= 12 |doi= 10.1016/j.str.2005.08.016 | pmid= 16338405 |url= http://www.sciencedirect.com/science/article/pii/S096921260500359X}}</ref> The lipoyl domain itself is attached by a flexible linker to the E2 core and the number of lipoyl domains varies from one to three for a given organism. The number of domains has been experimentally varied and seems to have little effect on growth until over nine are added, although more than three decreased activity of the complex.<ref>{{cite journal |last1= Machado |first1= RS |last2= Clark |first2= DP |last3= Guest |first3= JR |title= Construction and properties of pyruvate dehydrogenase complexes with up to nine lipoyl domains per lipoate acetyltransferase chain |journal= FEMS Microbiology Letters |year= 1992 |pages= 243–8 |volume= 79 |issue= 1–3 |doi= 10.1111/j.1574-6968.1992.tb14047.x |pmid= 1478460}}</ref> | |||
Lipoic acid serves as co-factor to the [[acetoin dehydrogenase]] complex catalyzing the conversion of acetoin (3-hydroxy-2-butanone) to acetaldehyde and [[acetyl coenzyme A]], in some bacteria, allowing acetoin to be used as the sole carbon source. | |||
The [[Glycine cleavage system]] differs from the other complexes, and has a different nomenclature. The individual components are free but it is sometimes incorrectly called a complex. In this system, the H protein is a free lipoyl domain with additional helices, the L protein is a dihydrolipoamide dehydrogenase, the P protein is the decarboxylase, and the T protein transfers the [[methylamine]] from lipoate to [[tetrahydrofolate]] (THF) yielding methylene-THF and ammonia. Methylene-THF is then used by serine hydroxymethyltransferase to synthesize [[serine]] from [[glycine]]. This system is part of plant [[photorespiration]].<ref>{{cite journal |last1= Douce |first1= R |last2= Bourguignon |first2= J |last3= Neuburger |first3= M |last4= Rebeille |first4= F |title= The glycine decarboxylase system: A fascinating complex |journal= [[Trends (journals)|Trends in Plant Science]] |year= 2001 |pages= 167–76 |volume= 6 |issue= 4 |doi= 10.1016/S1360-1385(01)01892-1 |pmid= 11286922}}</ref> | |||
===Biological sources and degradation=== | |||
Lipoic acid is present in almost all foods, but slightly more so in kidney, heart, liver, spinach, broccoli, and yeast extract.<ref>{{cite journal |last1= Durrani |first1= AI |last2= Schwartz |first2= H |last3= Nagl |first3= M |last4= Sontag |first4= G |title= Determination of free [alpha]-lipoic acid in foodstuffs by HPLC coupled with CEAD and ESI-MS |journal= [[Food Chemistry (journal)|Food Chemistry]] |date= October 2010 |pages= 38329–36 |volume= 120 |issue= 4 |doi= 10.1016/j.foodchem.2009.11.045}}</ref> Naturally occurring lipoic acid is always covalently bound and not readily available from dietary sources. In addition, the amount of lipoic acid present in dietary sources is very low. For instance, the purification of lipoic acid to determine its structure used an estimated 10 tons of liver residue, which yielded 30 mg of lipoic acid.<ref>{{cite journal |last= Reed |first= LJ |title= A trail of research from lipoic acid to alpha-keto acid dehydrogenase complexes |journal= [[Journal of Biological Chemistry]] |date= October 2001 |pages= 38329–36 |volume= 276 |issue= 42 |pmid= 11477096 |doi= 10.1074/jbc.R100026200 |url= http://www.jbc.org/content/276/42/38329.long}}</ref> As a result, all lipoic acid available as a supplement is chemically synthesized. | |||
Baseline levels (prior to supplementation) of RLA and R-DHLA have not been detected in human plasma.<ref>{{cite journal | doi = 10.1016/0928-0987(95)00045-3 |last1= Hermann |first1= R |year= 1996 |title= Enantioselective pharmacokinetics and bioavailability of different racemic formulations in healthy volunteers |journal= [[European Journal of Pharmaceutical Sciences]] |volume= 4 |issue= 3 |pages= 167–74 |last2= Niebch |first2= G |last3= Borbe |first3= HO |last4= Fieger |first4= H |last5= Ruus |first5= P |last6= Nowak |first6= H |last7= Riethmuller-Winzen |first7= H |last8= Peukert |first8= M |last9= Blume |first9= H |displayauthors= 4}}</ref> RLA has been detected at 12.3−43.1 ng/mL following acid hydrolysis, which releases protein-bound lipoic acid. Enzymatic hydrolysis of protein bound lipoic acid released 1.4−11.6 ng/mL and <1-38.2 ng/mL using subtilisin and alcalase, respectively.<ref>{{cite book |doi= 10.1016/S0076-6879(97)79019-0 |pmid= 9211267 |last1= Teichert |first1= J |last2= Preiss |first2= R |chapter= High-performance Liquid Chromatography Methods for Determination of Lipoic and Dihydrolipoic Acid in Human Plasma |volume= 279 |year= 1997 |pages= 159–66 |series= [[Methods in Enzymology]] |isbn= 9780121821807}}</ref><ref>{{cite journal |doi= 10.1016/0378-4347(95)00225-8 |pmid= 8581134 |last1= Teichert |first1= J |last2= Preiss |first2= R |title= Determination of lipoic acid in human plasma by high-performance liquid chromatography with electrochemical detection |journal= [[Journal of Chromatography B]] |volume= 672 |issue= 2 |date= October 1995 |pages=277–81}}</ref><ref>{{cite journal |pmid= 1490813 |last1= Teichert |first1= J |last2= Preiss |first2= R |title= HPLC-methods for determination of lipoic acid and its reduced form in human plasma |journal= International Journal of Clinical Pharmacology, Therapy, and Toxicology |volume= 30 |issue= 11 |date= November 1992 |pages= 511–2}}</ref> | |||
Digestive proteolytic enzymes cleave the R-lipoyllysine residue from the mitochondrial enzyme complexes derived from food but are unable to cleave the lipoic acid-<small>L</small>-[[lysine]] amide bond.<ref>{{cite journal |pmid= 9378235 |last1= Biewenga |first1= GP |last2= Haenen |first2= GR |last3= Bast |first3= A |title= The pharmacology of the antioxidant lipoic acid |journal= General Pharmacology |volume= 29 |issue= 3 |date= September 1997 |pages=315–31 |doi= 10.1016/S0306-3623(96)00474-0}}</ref> Both synthetic lipoamide and (''R'')-lipoyl-<small>L</small>-lysine are rapidly cleaved by serum lipoamidases, which release free (''R'')-lipoic acid and either <small>L</small>-lysine or ammonia.<ref name="Wada M 1961">{{cite journal |pmid= 14004240 |last1= Wada |first1= M |last2= Shigeta |first2= Y |last3= Inamori |first3= K |title= A study on the metabolism of lipoic acid and lipoamide |journal= Journal of Vitaminology |volume= 7 |issue= 3 |date= September 1961 |pages= 237–42 |doi= 10.5925/jnsv1954.7.237 |url= https://www.jstage.jst.go.jp/article/jnsv1954/7/3/7_3_237/_pdf}}</ref><ref name="Oizumi1989">{{cite journal |doi= 10.1016/0006-291X(89)92361-9 |pmid= 2502979 |last1= Oizumi |first1= J |last2= Hayakawa |first2= K |title= Liberation of lipoate by human serum lipoamidase from bovine heart pyruvate dehydrogenase |journal= [[Biochemical and Biophysical Research Communications]] |volume= 162 |issue= 2 |date= July 1989 |pages= 658–63}}</ref><ref name="Oizumi1989" /><ref>{{cite journal |last= Saito |first= J |year= 1960 |title= [The conversion of thioctamide to thioctic acid in biological systems. I. The thioctic active substances in rabbit serum after administration of thioctamide] |language= JA |url= http://ci.nii.ac.jp/naid/110002878000 |journal= Vitamin |volume= 21 |issue= 3 |pages= 359–63}}{{Closed access}}</ref><ref>{{cite journal |doi= 10.1016/0009-8981(90)90057-Y |pmid= 2127386 |last1= Backman-Gullers |first1= B |last2= Hannestad |first2= U |last3= Nilsson |first3= L |last4= Sorbo |first4= B |title= Studies on lipoamidase: Characterization of the enzyme in human serum and breast milk |journal= [[Clinica Chimica Acta]] |volume= 191 |issue= 1–2 |date= October 1990 |pages= 49–60}}</ref><ref>{{cite journal |doi= 10.1016/0009-8981(90)90313-H |pmid= 2225462 |last1= Garganta |first1= CL |last2= Wolf |first2= B |title= Lipoamidase activity in human serum is due to biotinidase |journal= [[Clinica Chimica Acta]] |volume= 189 |issue= 3 |date= August 1990 |pages= 313–25}}</ref> | |||
Little is known about the degradation and utilization of aliphatic sulfides such as lipoic acid, except for [[cysteine]]. Certain bacteria can use lipoic acid as a carbon, sulfur, and energy source. An abundant intermediate in lipoic acid degradation was the shorter bisnorlipoic acid.<ref>{{Cite journal |last1= Shih |first1= JC |last2= Rozo |first2= ML |last3= Wright |first3= LD |last4= McCormick |first4= DB |title= Characterization of the growth of ''Pseudomonas putida'' LP on lipoate and its analogues: Transport, oxidation, sulphur source, and enzyme induction |journal = [[Microbiology (journal)|Microbiology]] |volume= 86 |issue= 2 |pages= 217–27 |date= February 1975 |doi= 10.1099/00221287-86-2-217 |pmid= 1089758 |url= http://mic.sgmjournals.org/content/86/2/217.full.pdf}}</ref><ref>{{Cite journal |last1= Mansilla |first1= MC |last2= de Mendoza |first2= D |title= L-cysteine biosynthesis in ''Bacillus subtilis'': Identification, sequencing, and functional characterization of the gene coding for phosphoadenylylsulfate sulfotransferase |journal= [[Journal of Bacteriology]] |volume= 179 |issue= 3 |pages= 976–81 |date= February 1997 |doi= |pmid= 9006060 |pmc= 178787}}</ref> Although [[fatty acid degradation]] enzymes are likely involved, gene products responsible for use of lipoic acid as a sulfur source are unknown. | |||
Lipoic acid is metabolized in a variety of ways when given as a dietary supplement in mammals.<ref name="ReferenceA">{{Cite journal |last1= Schupke |first1= H |last2= Hempel |first2= R |last3= Peter |first3= G |last4= Hermann |first4= R |last5= Wessel |first5= K |last6= Engel |first6= J |last7= Kronbach |first7= T |displayauthors= 4 |title= New metabolic pathways of alpha-lipoic acid |journal= [[Drug Metabolism and Disposition]] |volume= 29 |issue= 6 |pages= 855–62 |date= June 2001 |doi= |pmid= 11353754}}</ref> Lipoic acid is partially degraded by a variety of transformations, which can occur in various combinations. Degradation to tetranorlipoic acid, oxidation of one or both of the sulfur atoms to the sulfoxide, and S-methylation of the sulfide were observed. Conjugation of unmodified lipoic acid to glycine was detected especially in mice.<ref name="ReferenceA"/> Degradation of lipoic acid is similar in humans, although it is not clear if the sulfur atoms become significantly oxidized.<ref>{{Cite journal |last1= Teichert |first1= J |last2= Hermann |first2= R |last3= Ruus |first3= P |last4= Preiss |first4= R |title= Plasma kinetics, metabolism, and urinary excretion of alpha-lipoic acid following oral administration in healthy volunteers |journal= [[The Journal of Clinical Pharmacology|Journal of Clinical Pharmacology]] |volume= 43 |issue= 11 |pages= 1257–67 |date= November 2003 |doi= 10.1177/0091270003258654 |pmid= 14551180}}</ref> Apparently mammals are not capable of utilizing lipoic acid as a sulfur source. | |||
==Chemical synthesis of lipoic acid== | |||
SLA did not exist prior to chemical synthesis in 1952.<ref>{{cite journal |last1= Hornberger |first1= CS |last2= Heitmiller |first2= RF |last3= Gunsalus |first3= IC |last4= Schnakenberg |first4= GHF |last5= Reed |first5= LJ |displayauthors= 4 |title= Synthesis of DL—lipoic acid |journal= [[Journal of the American Chemical Society]] |volume= 75 |issue= 6 |pages= 1273–7 |year= 1953 |doi= 10.1021/ja01102a003}}</ref><ref>{{cite journal |last1= Hornberger |first1= CS |last2= Heitmiller |first2= RF |last3= Gunsalus |first3= IC |last4= Schnakenberg |first4= GHF |last5= Reed |first5= LJ |displayauthors= 4 |title= Synthetic preparation of lipoic acid |journal= [[Journal of the American Chemical Society]] |volume= 74 |issue= 9 |page= 2382 |year= 1952 |doi= 10.1021/ja01129a511}}</ref> SLA is produced in equal amounts with RLA during achiral manufacturing processes. The racemic form was more widely used clinically in Europe and Japan in the 1950s to 1960s despite the early recognition that the various forms of LA are not bioequivalent.<ref name="Kleeman" /> The first synthetic procedures appeared for RLA and SLA in the mid-1950s.<ref>{{cite journal |pmid= 13294188 |last1= Fontanella |first1= L |title= Preparation of optical antipodes of alpha-lipoic acid |journal= Il Farmaco; edizione scientifica |volume= 10 |issue= 12 |year= 1955 |pages= 1043–5}}</ref><ref>{{cite journal |last1= Walton |first1= E |last2= Wagner |first2= AF |last3= Bachelor |first3= FW |last4= Peterson |first4= LH |last5= Holly |first5= FW |last6= Folkers |first6= K |displayauthors= 4 |title= Synthesis of (+)-lipoic acid and its optical antipode |journal= [[Journal of the American Chemical Society]] |volume= 77 |issue= 19 |pages= 5144–9 |year= 1955 |doi= 10.1021/ja01624a057|bibcode= 1955JAChS..77.1678G }}</ref><ref>{{cite journal |last1= Acker |first1= DS |last2= Wayne |first2= WJ |title= Optically active and radioactive α-lipoic acids |journal= [[Journal of the American Chemical Society]] |volume= 79 |issue= 24 |page= 6483 |year= 1957 |doi= 10.1021/ja01581a033}}</ref><ref>{{cite journal |pmid= 14207116 |last1= Deguchi |first1= Y |last2= Miura |first2= K |title= Studies on the synthesis of thioctic acid and its related compounds. XIV. Synthesis of (+)-thioctamide |journal= Yakugaku Zasshi |volume= 84 |date= June 1964 |pages= 562–3}}</ref> Advances in chiral chemistry led to more efficient technologies for manufacturing the single enantiomers by both [[classical resolution]] and [[asymmetric synthesis]] and the demand for RLA also grew at this time. In the 21st century, R/S-LA, RLA and SLA with high chemical and/or optical purities are available in industrial quantities. At the current time, most of the world supply of R/S-LA and RLA is manufactured in China and smaller amounts in Italy, Germany, and Japan. RLA is produced by modifications of a process first described by Georg Lang in a Ph.D. thesis and later patented by DeGussa.<ref>{{cite thesis |last= Lang |first= G |title= In Vitro Metabolism of a-Lipoic Acid Especially Taking Enantioselective Bio-transformation into Account |degree= Ph.D. |publisher= University of Münster |location= Münster, DE |year= 1992}}</ref><ref>{{cite patent |inventor1-last= Blaschke |inventor1-first= G |inventor2-last= Scheidmantel |inventor2-first= U |inventor3-last= Bethge |inventor3-first= H |inventor4-last= Moeller |inventor4-first= R |inventor5-last= Beisswenger |inventor6-last= Huthmacher |inventor6-first= T |title= Preparation and use of salts of the pure enantiomers of alpha-lipoic acid |country= US |number= 5281722 |status= patent |gdate= 1994-01-25 |assign1= DeGussa |fdate= 1992-11-12 |pridate= 1991-11-16 |postscript= .}}</ref> Although RLA is favored nutritionally due to its “vitamin-like” role in metabolism, both RLA and R/S-LA are widely available as dietary supplements. Both [[stereospecific]] and non-stereospecific reactions are known to occur ''in vivo'' and contribute to the mechanisms of action, but evidence to date indicates RLA may be the [[eutomer]] (the nutritionally and therapeutically preferred form).<ref name=Carlson08/><ref>{{cite journal |pmid= 11684397 |last1= Packer |first1= L |last2= Kraemer |first2= K |last3= Rimbach |first3= G |title= Molecular aspects of lipoic acid in the prevention of diabetes complications |journal= Nutrition |location= Burbank, CA |volume= 17 |issue= 10 |date= October 2001 |pages= 888–95 |doi= 10.1016/S0899-9007(01)00658-X}}</ref> | |||
==Pharmacology of lipoic acid== | |||
===Pharmacokinetics=== | |||
A 2007 human [[pharmacokinetic]] study of sodium RLA demonstrated the maximum concentration in plasma and bioavailability are significantly greater than the free acid form, and rivals plasma levels achieved by intravenous administration of the free acid form.<ref name="ReferenceB">{{cite journal |last1= Carlson |first1= DA |last2= Smith |first2= AR |last3= Fischer |first3= SJ |last4= Young |first4= KL |last5= Packer |first5= L |displayauthors= 4 |title= The plasma pharmacokinetics of R-(+)-lipoic acid administered as sodium R-(+)-lipoate to healthy human subjects |journal= Alternative Medicine Review |volume= 12 |issue= 4 |date= December 2007 |pages= 343–51 |pmid= 18069903 |url= http://www.altmedrev.com/publications/12/4/343.pdf}}</ref> Additionally, high plasma levels comparable to those in animal models where Nrf2 was activated were achieved.<ref name="ReferenceB"/> | |||
The various forms of LA are not bioequivalent.<ref name="Kleeman">{{cite conference |last1= Kleeman |first1= A |last2= Borbe |first2= HO |last3= Ulrich |first3= H |chapter= Thioctic Acid-Lipoic Acid |title= Thioctsäure: Neue Biochemische, Pharmakologische und Klinische Erkenntnisse zur Thioctsäure |trans_title= Thioctic Acid. New Biochemistry, Pharmacology and Findings from Clinical Practice with Thioctic Acid |pages= 11–26 |editor1-last= Borbe |editor1-first= HO |editor2-last= Ulrich |editor2-first= H |conference= Symposium at Wiesbaden, DE, 16-18 February 1989 |publicationdate= 1991 |publicationplace= Frankfurt, DE |publisher= Verlag |isbn= 9783891191255}}</ref> Very few studies compare individual enantiomers with racemic lipoic acid. It is unclear if twice as much racemic lipoic acid can replace RLA.<ref name="ReferenceB"/> | |||
The toxic dose of LA in cats is much lower than that in humans or dogs and produces hepatocellular toxicity.<ref>{{cite journal |last1= Hill |first1= AS |last2= Werner |first2=JA |last3= Rogers |first3= QR |last4= O'Neill |first4= SL |last5= Christopher |first5= MM |displayauthors= 4 |title= Lipoic acid is 10 times more toxic in cats than reported in humans, dogs or rats |journal= Journal of Animal Physiology and Animal Nutrition |volume= 88 |issue= 3–4 |date= April 2004 |pages= 150–6 |pmid= 15059240 |doi= 10.1111/j.1439-0396.2003.00472.x}}</ref> | |||
===[[Pharmacodynamics]]=== | |||
The mechanism and action of lipoic acid when supplied externally to an organism is controversial. Lipoic acid in a cell seems primarily to induce the oxidative stress response rather than directly scavenge free radicals. This effect is specific for RLA.<ref name= "Shay08"/> Despite the strongly reducing milieu, LA has been detected intracellularly in both oxidized and reduced forms.<ref name="Packer1995">{{cite journal |doi= 10.1016/0891-5849(95)00017-R |pmid= 7649494 |last1= Packer |first1= L |last2= Witt |first2= EH |last3= Tritschler |first3= HJ |title= Alpha-lipoic acid as a biological antioxidant |journal= [[Free Radical Biology and Medicine]] |volume= 19 |issue= 2 |date= August 1995 |pages= 227–50}}</ref> LA is reduced intracellularly to dihydrolipoic acid, which in cell culture regenerates by reduction of antioxidant radicals, such as vitamin C and vitamin E.<ref name="Packer1995" /> LA is able to scavenge reactive oxygen and reactive nitrogen species in a biochemical assay due to long incubation times, but there is little evidence this occurs within a cell or that radical scavenging contributes to the primary mechanisms of action of LA.<ref name= "Shay08"/><ref name="ReferenceC">{{cite journal |pmid= 19664690 |pmc= 2756298 |last1= Shay |first1= KP |doi= 10.1016/j.bbagen.2009.07.026 |last2= Moreau |first2= RF |last3= Smith |first3= EJ |last4= Smith |first4= AR |last5= Hagen |first5= TM |displayauthors= 4 | title = Alpha-lipoic acid as a dietary supplement: Molecular mechanisms and therapeutic potential |journal= [[Biochimica et Biophysica Acta]] |volume= 1790 |issue= 10 |date= October 2009 |pages= 1149–60}}</ref> The relatively good scavenging activity of LA toward hypochlorous acid (a bactericidal produced by neutrophils that may produce inflammation and tissue damage) is due to the strained conformation of the 5-membered dithiolane ring, which is lost upon reduction to DHLA. In cells, LA is reduced to dihydrolipoic acid, which is generally regarded as the more bioactive form of LA and the form responsible for most of the antioxidant effects.<ref>{{cite journal |last1= Haenen |first1= GRMM |last2= Bast |first2= A |year= 1991 |title= Scavenging of hypochlorous acid by lipoic acid |journal= [[Biochemical Pharmacology]] |pmid= 1659823 |volume= 42 |issue= 11 |pages= 2244–6 |doi= 10.1016/0006-2952(91)90363-A }}</ref> This theory has been challenged due to the high level of reactivity of the two free sulfhydryls, low intracellular concentrations of DHLA as well as the rapid methylation of one or both sulfhydryls, rapid side-chain oxidation to shorter metabolites and rapid efflux from the cell. Although both DHLA and LA have been found inside cells after administration, most intracellular DHLA probably exists as mixed disulfides with various cysteine residues from cytosolic and mitochondrial proteins.<ref name=Carlson08>{{cite book |last1= Carlson |first1= DA |last2= Young |first2= KL |last3= Fischer |first3= SJ |last4= Ulrich |first4= H |chapter= Ch. 10: An Evaluation of the Stability and Pharmacokinetics of R-lipoic Acid and R-Dihydrolipoic Acid Dosage Forms in Plasma from Healthy Human Subjects |pages= 235–70}} In {{harvnb|Packer|Patel|2008}}.</ref> Recent findings suggest therapeutic and anti-aging effects are due to modulation of signal transduction and gene transcription, which improve the antioxidant status of the cell. However, this likely occurs via pro-oxidant mechanisms, not by radical scavenging or reducing effects.<ref name= "Shay08"/><ref name="ReferenceC"/><ref name="Shay in Packer"/> | |||
All the [[disulfide]] forms of LA (R/S-LA, RLA and SLA) can be reduced to [[DHLA]] although both tissue specific and stereoselective (preference for one enantiomer over the other) reductions have been reported in model systems. At least two cytosolic enzymes, [[glutathione reductase]] (GR) and [[thioredoxin reductase]] (Trx1), and two mitochondrial enzymes, lipoamide dehydrogenase and [[thioredoxin reductase]] (Trx2), reduce LA. SLA is stereoselectively reduced by cytosolic GR whereas Trx1, Trx2 and lipoamide dehydrogenase stereoselectively reduce RLA. (''R'')-(+)-lipoic acid is enzymatically or chemically reduced to (''R'')-(-)-dihydrolipoic acid whereas (''S'')-(-)-lipoic acid is reduced to (''S'')-(+)-dihydrolipoic acid.<ref>{{cite journal |pmid= 8769129 |last1= Arnér |first1= ES |doi= 10.1006/bbrc.1996.1165 |last2= Nordberg |first2= J |last3= Holmgren |first3= A |title= Efficient reduction of lipoamide and lipoic acid by mammalian thioredoxin reductase |journal= [[Biochemical and Biophysical Research Communications]] |volume= 225 |issue= 1 |date= August 1996 |pages= 268–74}}</ref><ref>{{cite journal |doi= 10.1667/0033-7587(2003)159[0484:RROCDA]2.0.CO;2 |pmid= 12643793 |last1= Biaglow |first1= JE |last2= Ayene |first2= IS |last3= Koch |first3= CJ |last4= Donahue |first4= J |last5= Stamato |first5= TD |last6= Mieyal |first6= JJ |last7= Tuttle |first7= SW |displayauthors= 4 |title= Radiation response of cells during altered protein thiol redox |journal= Radiation Research |volume= 159 |issue= 4 |date= April 2003 |pages= 484–94 }}</ref><ref>{{cite journal |doi= 10.1016/S0891-5849(96)00400-5 |pmid= 8981046 |last1= Haramaki |first1= N |last2= Han |first2= D |last3= Handelman |first3= GJ |last4= Tritschler |first4= HJ |last5= Packer |first5= L |displayauthors= 4 |title= Cytosolic and mitochondrial systems for NADH- and NADPH-dependent reduction of alpha-lipoic acid |journal= [[Free Radical Biology and Medicine]] |volume= 22 |issue= 3 |year= 1997 |pages= 535–42}}</ref><ref>{{cite journal |doi= 10.1016/0006-2952(95)00084-D |pmid= 7632170 |last1= Constantinescu |first1= A |last2= Pick |first2= U |last3= Handelman |first3= GJ |last4= Haramaki |first4= N |last5= Han |first5= D |last6= Podda |first6= M |last7= Tritschler |first7= HJ |last8= Packer |first8= L |displayauthors= 4 |title= Reduction and transport of lipoic acid by human erythrocytes |journal= [[Biochemical Pharmacology]] |volume= 50 |issue= 2 |date= July 1995 |pages= 253–61}}</ref><ref>{{cite journal |pmid= 16650819 |last1= May |first1= JM |doi= 10.1016/j.bbrc.2006.04.065 |last2= Qu |first2= ZC |last3= Nelson |first3= DJ |title= Cellular disulfide-reducing capacity: An integrated measure of cell redox capacity |journal= [[Biochemical and Biophysical Research Communications]] |volume= 344 |issue= 4 |date= June 2006 |pages= 1352–9}}</ref><ref>{{cite journal |doi= 10.1016/S0891-5849(02)00862-6 |pmid= 12086686 |last1= Jones |first1= W |last2= Li |first2= X |last3= Qu |first3= ZC |last4= Perriott |first4= L |last5= Whitesell |first5= RR |last6= May |first6= JM |displayauthors= 4 |title= Uptake, recycling, and antioxidant actions of alpha-lipoic acid in endothelial cells |journal= [[Free Radical Biology and Medicine]] |volume= 33 |issue= 1 |date= July 2002 |pages= 83–93}}</ref><ref>{{cite journal |last1= Schempp |first1= H |last2= Ulrich |first2= H |last3= Elstner |first3= EF |title= Stereospecific reduction of R(+)-thioctic acid by porcine heart lipoamide dehydrogenase/diaphorase |journal= [[Zeitschrift für Naturforschung C]] |volume= 49 |issue= 9–10 |pages= 691–2 |year= 1994 |pmid= 7945680 }}</ref> Dihydrolipoic acid (DHLA) can also form intracellularly and extracellularly via non-enzymatic, [[thiol-disulfide exchange reactions]].<ref>{{cite book |last1= Biewenga |first1= GP |last2= Haenen |first2= GRMM |last3= Bast |first3= A |chapter= Ch. 1: An Overview of Lipoate Chemistry |editor1-last= Fuchs |editor1-first= J |editor2-last= Packer |editor2-first= L |editor3-last= Zimmer |editor3-first= G |title= Lipoic Acid In Health & Disease |publisher= [[CRC Press]] |year= 1997 |pages= [http://books.google.com/books?id=ksWdMbxa5FkC&pg=PA1 1–32] |isbn= 9780824700935}}</ref> | |||
== | RLA may function ''in vivo'' like a B-vitamin and at higher doses like plant-derived nutrients, such as [[curcumin]], [[sulphoraphane]], [[resveratrol]], and other nutritional substances that induce [[Drug metabolism#Phase II – conjugation|phase II detoxification enzymes]], thus acting as cytoprotective agents.<ref name="Shay in Packer">{{cite book |last1= Shay |first1= KP |last2= Shenvi |first2= S |last3= Hagen |first3= TM |chapter= Ch. 14 Lipoic Acid as an Inducer of Phase II Detoxification Enzymes Through Activation of Nr-f2 Dependent Gene Expression |pages= 349–71}} In {{harvnb|Packer|Patel|2008}}.</ref><ref>{{cite journal |last1=Lii |first1= CK |last2= Liu |first2= KL |last3= Cheng |first3= YP |last4= Lin |first4= AH |last5= Chen |first5= HW |last6= Tsai |first6= CW |displayauthors= 4 |title= Sulforaphane and alpha-lipoic acid upregulate the expression of the pi class of glutathione S-transferase through c-jun and Nrf2 activation |journal= [[Journal of Nutrition]] |volume= 140 |issue= 5 |pages= 885–92 |date= May 2010 |pmid= 20237067 |doi= 10.3945/jn.110.121418 |url= http://jn.nutrition.org/cgi/pmidlookup?view=long&pmid=20237067}}</ref> This stress response indirectly improves the antioxidant capacity of the cell.<ref name= "Shay08">{{cite journal |pmid= 18409172 |last1= Shay |first1= KP |doi= 10.1002/iub.40 |last2= Moreau |first2= RF |last3= Smith |first3= EJ |last4= Hagen |first4= TM |title= Is alpha-lipoic acid a scavenger of reactive oxygen species in vivo? Evidence for its initiation of stress signaling pathways that promote endogenous antioxidant capacity |journal= IUBMB Life |volume= 60 |issue= 6 |date= June 2008 |pages= 362–7 |url= http://onlinelibrary.wiley.com/doi/10.1002/iub.40/full}}</ref> | ||
''' | The (''S'')-enantiomer of LA was shown to be toxic when administered to thiamine-deficient rats.<ref>{{cite journal |doi= 10.1016/0003-9861(60)90051-5 |pmid= 13825981 |last1= Gal |first1= EM |last2= Razevska |first2= DE |title= Studies on the in vivo metabolism of lipoic acid. 1. The fate of DL-lipoic acid-S35 in normal and thiamine-deficient rats |journal= [[Archives of Biochemistry and Biophysics]] |volume= 89 |date= August 1960 |pages= 253–61 |issue= 2}}</ref><ref name="Gal1965">{{cite journal |doi= 10.1038/207535a0 |pmid= 5328673 |last1= Gal |first1= EM |title= Reversal of selective toxicity of (-)-alpha-lipoic acid by thiamine in thiamine-deficient rats |journal= [[Nature (journal)|Nature]] |volume= 207 |issue= 996 |date= July 1965 |page= 535|bibcode= 1965Natur.207..535G }}</ref> | ||
Several studies have demonstrated that SLA either has lower activity than RLA or interferes with the specific effects of RLA by [[competitive inhibition]].<ref>{{cite patent |inventor1-last= Ulrich |inventor1-first= H |inventor2-last= Weischer |inventor2-first= CH |inventor3-last= Engel |inventor3-first= J |inventor4-last= Hettche |inventor4-first= H |title= Pharmaceutical compositions containing R-alpha-lipoic acid or S-alpha.-lipoic acid as active ingredient |country= US |number= 6271254 |gdate= 2001-08-07 |status= patent |assign1= ASTA Pharma |fdate= 1998-02-02 |pridate= 1989-11-09 |postscript= .}}</ref><ref>{{cite journal |pmid= 8673020 |last1= Kilic |first1= F |last2= Handelman |first2= GJ |last3= Serbinova |first3= E |last4= Packer |first4= L |last5= Trevithick |first5= JR |displayauthors= 4 |title= Modelling cortical cataractogenesis 17: In vitro effect of a-lipoic acid on glucose-induced lens membrane damage, a model of diabetic cataractogenesis |journal= Biochemistry and Molecular Biology International |volume= 37 |issue= 2 |date= October 1995 |pages= 361–70}}</ref><ref>{{cite conference |last1= Artwohl |first1= M |last2= Schmetterer |first2= L |last3= Rainer |first3= G |last4= unknown |displayauthors= 3 |date= September 2000|contribution= Modulation by antioxidants of endothelial apoptosis, proliferation, & associated gene/protein expression |conference= 36th Annual Meeting of the European Association for the Study of Diabetes, 17-21 September 2000, Jerusalem, Israel. |journal= [[Diabetologia]] |volume= 43 |issue= Suppl 1 |page= Abs 274 |publicationdate= August 2000 |nopp= yes |pmid= 11008622}}</ref><ref>{{cite journal |pmid= 9252495 |last1= Streeper |first1= RS |last2= Henriksen |first2= EJ |last3= Jacob |first3= S |last4= Hokama |first4= JY |last5= Fogt |first5= DL |last6= Tritschler |first6= HJ |displayauthors= 4 |title= Differential effects of lipoic acid stereoisomers on glucose metabolism in insulin-resistant skeletal muscle |journal= [[American Journal of Physiology|AJP - Endocrinology and Metabolism]] |volume= 273 |issue= 1 Pt 1 |date= July 1997 |pages= E185–91}}</ref><ref>{{cite journal |pmid= 14991456 |last1= Frölich |first1= L |last2= Götz |first2= ME |last3= Weinmüller |first3= M |last4= Youdim |first4= MB |last5= Barth |first5= N |last6= Dirr |first6= A |last7= Gsell |first7= W |last8= Jellinger |first8= K |last9= Beckmann |first9= H |last10= Riederer |first10= P |displayauthors= 4 |title= (r)-, but not (s)-alpha lipoic acid stimulates deficient brain pyruvate dehydrogenase complex in vascular dementia, but not in Alzheimer dementia |journal = Journal of Neural Transmission |volume= 111 |issue= 3 |date= March 2004 |pages= 295–310 |doi= 10.1007/s00702-003-0043-5}}</ref> | |||
== | ==Uses== | ||
R/S-LA and RLA are widely available as over-the-counter nutritional supplements in the United States in the form of capsules, tablets, and aqueous liquids, and have been marketed as [[antioxidants]]. This label has recently been challenged.<ref name= "Shay08"/> In Japan, LA is marketed primarily as a "weight loss" and "energy" supplement.{{citation needed|date=April 2014}} The relationships between supplemental doses and therapeutic doses have not been clearly defined. Because lipoic acid is not an [[essential nutrient]], no [[Recommended Daily Allowance]] (RDA) has been established. In Germany, LA is approved as a drug against diabetes comorbidities since 1966 and available by prescription.<ref name=Ziegle>{{Cite journal|last1=Ziegle|first1=D.|last2=Reljanovic|first2=M|last3=Mehnert|first3=H|last4=Gries|first4=F. A.|title=α-Lipoic acid in the treatment of diabetic polyneuropathy in Germany|journal=Experimental and Clinical Endocrinology & Diabetes|volume=107|issue=7|pages=421–30|url=http://www.ncbi.nlm.nih.gov/pubmed/10595592|publisher=J. A. Barth Verlag in Georg Thieme Verlag KG Stuttgart|accessdate=28 August 2014|year=1999|pmid=10595592|doi=10.1055/s-0029-1212132}}</ref> | |||
[[File:(RS)-Lipoic_Acid_Structural_Formulea_V.1.svg.png|thumb|260px|(''R'')-Lipoic acid (RLA, top) and (''S'')-lipoic acid (SLA, down). A 1:1 mixture ([[racemate]]) of (''R'')- and (''S'')-lipoic acid is called (''RS'')-lipoic acid or (±)-lipoic acid (R/S-LA).]] | |||
== | ==Clinical research== | ||
* According to the [[American Cancer Society]], "there is no reliable scientific evidence at this time that lipoic acid prevents the development or spread of cancer".<ref>{{cite web|url=http://www.cancer.org/treatment/treatmentsandsideeffects/complementaryandalternativemedicine/pharmacologicalandbiologicaltreatment/lipoic-acid|title=Lipoic Acid|date=November 2008|publisher=[[American Cancer Society]]|accessdate=5 October 2013}}</ref> | |||
* For peripheral [[diabetic neuropathy]], intravenous administration of alpha lipoic acid leads to a short-term improvement, but there is no good evidence of meaningful benefit when taking it by mouth.<ref name=diabetes>{{cite journal |author=Mijnhout GS, Kollen BJ, Alkhalaf A, Kleefstra N, Bilo HJ |title=Alpha lipoic Acid for symptomatic peripheral neuropathy in patients with diabetes: a meta-analysis of randomized controlled trials |journal=Int J Endocrinol |volume=2012 |issue= |pages=456279 |year=2012 |pmid=22331979 |pmc=3272801 |doi=10.1155/2012/456279 |type=Meta-analysis|last2=Kollen |last3=Alkhalaf |last4=Kleefstra |last5=Bilo }}</ref> | |||
* A review of literature, using studies available from January 2008, did not find any randomized controlled trials using lipoic acid to treat [[dementia]]. Due to the absence of evidence it could not support lipoic acid for the treatment of any form of Dementia.<ref name=dementia>{{cite journal |author=Sauer J, Tabet N, Howard R |title=Alpha lipoic acid for dementia |journal=Cochrane Database Syst Rev |volume= |issue=1 |pages=CD004244 |year=2008 |pmid=14974062 |doi=10.1002/14651858.CD004244.pub2 |type=Systematic review|last2=Tabet |last3=Howard |last4=Howard }}</ref> | |||
* There is weak evidence alpha lipoic acid may help with the management of [[burning mouth syndrome]].<ref>{{cite journal |author=Patton LL, Siegel MA, Benoliel R, De Laat A |title=Management of burning mouth syndrome: systematic review and management recommendations |journal=Oral Surg Oral Med Oral Pathol Oral Radiol Endod |volume=103 Suppl |issue= |pages=S39.e1–13 |date=March 2007 |pmid=17379153 |doi=10.1016/j.tripleo.2006.11.009 |type=Systematic review|last2=Siegel |last3=Benoliel |last4=De Laat }}</ref> | |||
== | * There is no evidence alpha lipoic acid helps people with mitochondrial disorders.<ref name=mito>{{cite journal |author=Pfeffer G, Majamaa K, Turnbull DM, Thorburn D, Chinnery PF |title=Treatment for mitochondrial disorders |journal=Cochrane Database Syst Rev |volume=4 |issue= |pages=CD004426 |year=2012 |pmid=22513923 |doi=10.1002/14651858.CD004426.pub3 |type=Systematic review|last2=Majamaa |last3=Turnbull |last4=Thorburn |last5=Chinnery }}</ref> | ||
* There is limited evidence lipoic acid may have potential as a drug for treating [[multiple sclerosis]].<ref>{{cite journal |author=Salinthone S, Yadav V, Bourdette DN, Carr DW |title=Lipoic acid: a novel therapeutic approach for multiple sclerosis and other chronic inflammatory diseases of the CNS |journal=Endocr Metab Immune Disord Drug Targets |volume=8 |issue=2 |pages=132–42 |date=June 2008 |pmid=18537699 |type=Report |quote=Based on EAE studies and the preliminary results from this clinical trial, LA appears to have a potential of a useful drug in treating MS. |doi=10.2174/187153008784534303|last2=Yadav |last3=Bourdette |last4=Carr }}</ref> | |||
| doi= 10. | |||
=== | ===Clinical Adverse effects=== | ||
Side effects of alpha lipoic acid may include headache, tingling or a "pins and needles" sensation, skin rash, or muscle cramps. There have been a few reports in Japan of a rare condition called insulin autoimmune syndrome in people using alpha lipoic acid.<ref>{{cite journal |last1= Ishida |first1= Y |last2= Ohara |first2= T |last3= Okuno |first3= Y |last4= Ito |first4= T |last5= Hirota |first5= Y |last6= Furukawa |first6= K |last7= Sakaguchi |first7= K |last8= Ogawa |first8= W |last9= Kasuga |first9= M |displayauthors= 4 |title= Alpha-lipoic acid and insulin autoimmune syndrome |journal= [[Diabetes Care]] |volume= 30 |issue= 9 |year= 2007 |pages= 2240–1 |pmid= 17586737 |doi= 10.2337/dc07-0689 |url= http://care.diabetesjournals.org/content/30/9/2240.full}}</ref> The condition causes hypoglycemia and antibodies directed against the body's own insulin without previous insulin therapy. The safety of alpha lipoic acid in pregnant or nursing women, children, or people with kidney or liver disease is unknown.{{citation needed|date=April 2014}} | |||
== | ==Other lipoic acids== | ||
*β-lipoic acid is a thiosulfinate of α-lipoic acid | |||
==References== | ==References== | ||
{{Reflist|2}} | {{Reflist|2}} | ||
*{{cite book |editor1-last= Packer |editor1-first= Lester |editor2-last= Patel |editor2-first= Mulchand S. |title= Lipoic Acid: Energy Production, Antioxidant Activity and Health Effects |publisher= [[CRC Press]] |location= Boca Raton, FL |year= 2008 |isbn= 1420045377 |ref= harv}} | |||
== | ==Further reading== | ||
*{{Pauling|id=othernuts/la | title=Lipoic Acid | author=Jane Higdon}} | *{{Pauling|id=othernuts/la | title=Lipoic Acid | author=Jane Higdon}} | ||
{{Enzyme cofactors}} | {{Enzyme cofactors}} | ||
[[Category: | {{Dietary supplement}} | ||
{{Nootropics}} | |||
[[Category:Antioxidants]] | |||
[[Category:Carboxylic acids]] | [[Category:Carboxylic acids]] | ||
[[Category:Cofactors]] | [[Category:Cofactors]] | ||
[[Category:Eli Lilly and Company]] | |||
[[ | [[Category:Organic disulfides]] | ||
[[ | [[Category:Dithiolanes]] | ||
[[ | |||
Latest revision as of 18:10, 14 April 2015
| |
| |
| |
| Names | |
|---|---|
| IUPAC name
(R)-5-(1,2-dithiolan-3-yl)pentanoic acid
| |
| Other names
α-Lipoic acid (alpha lipoic acid); Thioctic acid; 6,8-Dithiooctanoic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | Lua error in Module:Wikidata at line 879: attempt to index field 'wikibase' (a nil value). Lua error in Module:Wikidata at line 879: attempt to index field 'wikibase' (a nil value). |
| KEGG | |
| MeSH | Lipoic+acid |
PubChem CID
|
|
| |
| |
| Properties | |
| C8H14O2S2 | |
| Molar mass | 206.32 g·mol−1 |
| Related compounds | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
|
WikiDoc Resources for Lipoic acid |
|
Articles |
|---|
|
Most recent articles on Lipoic acid Most cited articles on Lipoic acid |
|
Media |
|
Powerpoint slides on Lipoic acid |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Lipoic acid at Clinical Trials.gov Clinical Trials on Lipoic acid at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Lipoic acid
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Lipoic acid Discussion groups on Lipoic acid Patient Handouts on Lipoic acid Directions to Hospitals Treating Lipoic acid Risk calculators and risk factors for Lipoic acid
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Lipoic acid |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Lipoic acid (LA), also known as α-lipoic acid and alpha lipoic acid (ALA) and thioctic acid is an organosulfur compound derived from octanoic acid. ALA is made in animals normally, and is essential for aerobic metabolism. It is also manufactured and is available as a dietary supplement in some countries where it is marketed as an antioxidant, and is available as a pharmaceutical drug in other countries.
Physical and chemical properties
Lipoic acid (LA), also known as α-lipoic acid[1] and alpha lipoic acid (ALA) and thioctic acid[2] is an organosulfur compound derived from octanoic acid. LA contains two sulfur atoms (at C6 and C8) connected by a disulfide bond and is thus considered to be oxidized although either sulfur atom can exist in higher oxidation states.
The carbon atom at C6 is chiral and the molecule exists as two enantiomers (R)-(+)-lipoic acid (RLA) and (S)-(-)-lipoic acid (SLA) and as a racemic mixture (R/S)-lipoic acid (R/S-LA).
LA appears physically as a yellow solid and structurally contains a terminal carboxylic acid and a terminal dithiolane ring.[citation needed]
For use in dietary supplement materials and compounding pharmacies, the USP has established an official monograph for R/S-LA.[3]
Biological function
"Lipoate" is the conjugate base of lipoic acid, and the most prevalent form of LA under physiologic conditions. Most endogenously produced RLA is not “free” because octanoic acid, the precursor to RLA, is bound to the enzyme complexes prior to enzymatic insertion of the sulfur atoms. As a cofactor, RLA is covalently attached by an amide bond to a terminal lysine residue of the enzyme’s lipoyl domains. One of the most studied roles of RLA is as a cofactor of the pyruvate dehydrogenase complex (PDC or PDHC), though it is a cofactor in other enzymatic systems as well (described below).
Only the (R)-(+)-enantiomer (RLA) exists in nature and is essential for aerobic metabolism because RLA is an essential cofactor of many enzyme complexes.[4]
Biosynthesis and attachment
The precursor to lipoic acid, octanoic acid, is made via fatty acid biosynthesis in the form of octanoyl-acyl carrier protein. In eukaryotes, a second fatty acid biosynthetic pathway in mitochondria is used for this purpose.[5][6] The octanoate is transferred as a thioester of acyl carrier protein from fatty acid biosynthesis to an amide of the lipoyl domain protein by an enzyme called an octanoyltransferase. Two hydrogens of octanoate are replaced with sulfur groups via a radical SAM mechanism, by lipoyl synthase [7] As a result, lipoic acid is synthesized attached to proteins and no free lipoic acid is produced. Lipoic acid can be removed whenever proteins are degraded and by action of the enzyme lipoamidase.[8] Free lipoate can be used by some organisms an enzyme called lipoate protein ligase that attaches it covalently to the correct protein. The ligase activity of this enzyme requires ATP.[9]
Enzymatic Activity
Lipoic acid is cofactor for at least five enzyme systems. Two of these are in the citric acid cycle through which many organisms turn nutrients into energy. Lipoylated enzymes have lipoic acid attached to them covalently. The lipoyl group transfers acyl groups in 2-oxoacid dehydrogenase complexes, and methylamine group in the glycine cleavage complex or glycine dehydrogenase.
2-Oxoacid dehydrogenase transfer reactions occur by a similar mechanism in:
- the pyruvate dehydrogenase complex
- the α-ketoglutarate dehydrogenase or 2-oxoglutarate dehydrogenase complex
- the branched-chain oxoacid dehydrogenase (BCDH) complex
- the acetoin dehydrogenase complex.
The most-studied of these is the pyruvate dehydrogenase complex. These complexes have three central subunits: E1-3, which are the decarboxylase, lipoyl transferase, and dihydrolipoamide dehydrogenase, respectively. These complexes have a central E2 core and the other subunits surround this core to form the complex. In the gap between these two subunits, the lipoyl domain ferries intermediates between the active sites.[10][11] The lipoyl domain itself is attached by a flexible linker to the E2 core and the number of lipoyl domains varies from one to three for a given organism. The number of domains has been experimentally varied and seems to have little effect on growth until over nine are added, although more than three decreased activity of the complex.[12]
Lipoic acid serves as co-factor to the acetoin dehydrogenase complex catalyzing the conversion of acetoin (3-hydroxy-2-butanone) to acetaldehyde and acetyl coenzyme A, in some bacteria, allowing acetoin to be used as the sole carbon source.
The Glycine cleavage system differs from the other complexes, and has a different nomenclature. The individual components are free but it is sometimes incorrectly called a complex. In this system, the H protein is a free lipoyl domain with additional helices, the L protein is a dihydrolipoamide dehydrogenase, the P protein is the decarboxylase, and the T protein transfers the methylamine from lipoate to tetrahydrofolate (THF) yielding methylene-THF and ammonia. Methylene-THF is then used by serine hydroxymethyltransferase to synthesize serine from glycine. This system is part of plant photorespiration.[13]
Biological sources and degradation
Lipoic acid is present in almost all foods, but slightly more so in kidney, heart, liver, spinach, broccoli, and yeast extract.[14] Naturally occurring lipoic acid is always covalently bound and not readily available from dietary sources. In addition, the amount of lipoic acid present in dietary sources is very low. For instance, the purification of lipoic acid to determine its structure used an estimated 10 tons of liver residue, which yielded 30 mg of lipoic acid.[15] As a result, all lipoic acid available as a supplement is chemically synthesized.
Baseline levels (prior to supplementation) of RLA and R-DHLA have not been detected in human plasma.[16] RLA has been detected at 12.3−43.1 ng/mL following acid hydrolysis, which releases protein-bound lipoic acid. Enzymatic hydrolysis of protein bound lipoic acid released 1.4−11.6 ng/mL and <1-38.2 ng/mL using subtilisin and alcalase, respectively.[17][18][19]
Digestive proteolytic enzymes cleave the R-lipoyllysine residue from the mitochondrial enzyme complexes derived from food but are unable to cleave the lipoic acid-L-lysine amide bond.[20] Both synthetic lipoamide and (R)-lipoyl-L-lysine are rapidly cleaved by serum lipoamidases, which release free (R)-lipoic acid and either L-lysine or ammonia.[21][22][22][23][24][25]
Little is known about the degradation and utilization of aliphatic sulfides such as lipoic acid, except for cysteine. Certain bacteria can use lipoic acid as a carbon, sulfur, and energy source. An abundant intermediate in lipoic acid degradation was the shorter bisnorlipoic acid.[26][27] Although fatty acid degradation enzymes are likely involved, gene products responsible for use of lipoic acid as a sulfur source are unknown.
Lipoic acid is metabolized in a variety of ways when given as a dietary supplement in mammals.[28] Lipoic acid is partially degraded by a variety of transformations, which can occur in various combinations. Degradation to tetranorlipoic acid, oxidation of one or both of the sulfur atoms to the sulfoxide, and S-methylation of the sulfide were observed. Conjugation of unmodified lipoic acid to glycine was detected especially in mice.[28] Degradation of lipoic acid is similar in humans, although it is not clear if the sulfur atoms become significantly oxidized.[29] Apparently mammals are not capable of utilizing lipoic acid as a sulfur source.
Chemical synthesis of lipoic acid
SLA did not exist prior to chemical synthesis in 1952.[30][31] SLA is produced in equal amounts with RLA during achiral manufacturing processes. The racemic form was more widely used clinically in Europe and Japan in the 1950s to 1960s despite the early recognition that the various forms of LA are not bioequivalent.[32] The first synthetic procedures appeared for RLA and SLA in the mid-1950s.[33][34][35][36] Advances in chiral chemistry led to more efficient technologies for manufacturing the single enantiomers by both classical resolution and asymmetric synthesis and the demand for RLA also grew at this time. In the 21st century, R/S-LA, RLA and SLA with high chemical and/or optical purities are available in industrial quantities. At the current time, most of the world supply of R/S-LA and RLA is manufactured in China and smaller amounts in Italy, Germany, and Japan. RLA is produced by modifications of a process first described by Georg Lang in a Ph.D. thesis and later patented by DeGussa.[37][38] Although RLA is favored nutritionally due to its “vitamin-like” role in metabolism, both RLA and R/S-LA are widely available as dietary supplements. Both stereospecific and non-stereospecific reactions are known to occur in vivo and contribute to the mechanisms of action, but evidence to date indicates RLA may be the eutomer (the nutritionally and therapeutically preferred form).[39][40]
Pharmacology of lipoic acid
Pharmacokinetics
A 2007 human pharmacokinetic study of sodium RLA demonstrated the maximum concentration in plasma and bioavailability are significantly greater than the free acid form, and rivals plasma levels achieved by intravenous administration of the free acid form.[41] Additionally, high plasma levels comparable to those in animal models where Nrf2 was activated were achieved.[41]
The various forms of LA are not bioequivalent.[32] Very few studies compare individual enantiomers with racemic lipoic acid. It is unclear if twice as much racemic lipoic acid can replace RLA.[41]
The toxic dose of LA in cats is much lower than that in humans or dogs and produces hepatocellular toxicity.[42]
Pharmacodynamics
The mechanism and action of lipoic acid when supplied externally to an organism is controversial. Lipoic acid in a cell seems primarily to induce the oxidative stress response rather than directly scavenge free radicals. This effect is specific for RLA.[1] Despite the strongly reducing milieu, LA has been detected intracellularly in both oxidized and reduced forms.[43] LA is reduced intracellularly to dihydrolipoic acid, which in cell culture regenerates by reduction of antioxidant radicals, such as vitamin C and vitamin E.[43] LA is able to scavenge reactive oxygen and reactive nitrogen species in a biochemical assay due to long incubation times, but there is little evidence this occurs within a cell or that radical scavenging contributes to the primary mechanisms of action of LA.[1][44] The relatively good scavenging activity of LA toward hypochlorous acid (a bactericidal produced by neutrophils that may produce inflammation and tissue damage) is due to the strained conformation of the 5-membered dithiolane ring, which is lost upon reduction to DHLA. In cells, LA is reduced to dihydrolipoic acid, which is generally regarded as the more bioactive form of LA and the form responsible for most of the antioxidant effects.[45] This theory has been challenged due to the high level of reactivity of the two free sulfhydryls, low intracellular concentrations of DHLA as well as the rapid methylation of one or both sulfhydryls, rapid side-chain oxidation to shorter metabolites and rapid efflux from the cell. Although both DHLA and LA have been found inside cells after administration, most intracellular DHLA probably exists as mixed disulfides with various cysteine residues from cytosolic and mitochondrial proteins.[39] Recent findings suggest therapeutic and anti-aging effects are due to modulation of signal transduction and gene transcription, which improve the antioxidant status of the cell. However, this likely occurs via pro-oxidant mechanisms, not by radical scavenging or reducing effects.[1][44][46]
All the disulfide forms of LA (R/S-LA, RLA and SLA) can be reduced to DHLA although both tissue specific and stereoselective (preference for one enantiomer over the other) reductions have been reported in model systems. At least two cytosolic enzymes, glutathione reductase (GR) and thioredoxin reductase (Trx1), and two mitochondrial enzymes, lipoamide dehydrogenase and thioredoxin reductase (Trx2), reduce LA. SLA is stereoselectively reduced by cytosolic GR whereas Trx1, Trx2 and lipoamide dehydrogenase stereoselectively reduce RLA. (R)-(+)-lipoic acid is enzymatically or chemically reduced to (R)-(-)-dihydrolipoic acid whereas (S)-(-)-lipoic acid is reduced to (S)-(+)-dihydrolipoic acid.[47][48][49][50][51][52][53] Dihydrolipoic acid (DHLA) can also form intracellularly and extracellularly via non-enzymatic, thiol-disulfide exchange reactions.[54]
RLA may function in vivo like a B-vitamin and at higher doses like plant-derived nutrients, such as curcumin, sulphoraphane, resveratrol, and other nutritional substances that induce phase II detoxification enzymes, thus acting as cytoprotective agents.[46][55] This stress response indirectly improves the antioxidant capacity of the cell.[1]
The (S)-enantiomer of LA was shown to be toxic when administered to thiamine-deficient rats.[56][57]
Several studies have demonstrated that SLA either has lower activity than RLA or interferes with the specific effects of RLA by competitive inhibition.[58][59][60][61][62]
Uses
R/S-LA and RLA are widely available as over-the-counter nutritional supplements in the United States in the form of capsules, tablets, and aqueous liquids, and have been marketed as antioxidants. This label has recently been challenged.[1] In Japan, LA is marketed primarily as a "weight loss" and "energy" supplement.[citation needed] The relationships between supplemental doses and therapeutic doses have not been clearly defined. Because lipoic acid is not an essential nutrient, no Recommended Daily Allowance (RDA) has been established. In Germany, LA is approved as a drug against diabetes comorbidities since 1966 and available by prescription.[63]
Clinical research
- According to the American Cancer Society, "there is no reliable scientific evidence at this time that lipoic acid prevents the development or spread of cancer".[64]
- For peripheral diabetic neuropathy, intravenous administration of alpha lipoic acid leads to a short-term improvement, but there is no good evidence of meaningful benefit when taking it by mouth.[65]
- A review of literature, using studies available from January 2008, did not find any randomized controlled trials using lipoic acid to treat dementia. Due to the absence of evidence it could not support lipoic acid for the treatment of any form of Dementia.[66]
- There is weak evidence alpha lipoic acid may help with the management of burning mouth syndrome.[67]
- There is no evidence alpha lipoic acid helps people with mitochondrial disorders.[68]
- There is limited evidence lipoic acid may have potential as a drug for treating multiple sclerosis.[69]
Clinical Adverse effects
Side effects of alpha lipoic acid may include headache, tingling or a "pins and needles" sensation, skin rash, or muscle cramps. There have been a few reports in Japan of a rare condition called insulin autoimmune syndrome in people using alpha lipoic acid.[70] The condition causes hypoglycemia and antibodies directed against the body's own insulin without previous insulin therapy. The safety of alpha lipoic acid in pregnant or nursing women, children, or people with kidney or liver disease is unknown.[citation needed]
Other lipoic acids
- β-lipoic acid is a thiosulfinate of α-lipoic acid
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Shay, KP; Moreau, RF; Smith, EJ; Hagen, TM (June 2008). "Is alpha-lipoic acid a scavenger of reactive oxygen species in vivo? Evidence for its initiation of stress signaling pathways that promote endogenous antioxidant capacity". IUBMB Life. 60 (6): 362–7. doi:10.1002/iub.40. PMID 18409172.
- ↑ Reljanovic, M; Reichel, G; Rett, K; Lobisch, M; et al. (September 1999). "Treatment of diabetic polyneuropathy with the antioxidant thioctic acid (alpha-lipoic acid): A two year multicenter randomized double-blind placebo-controlled trial (ALADIN II). Alpha Lipoic Acid in Diabetic Neuropathy". Free Radical Research. 31 (3): 171–9. doi:10.1080/10715769900300721. PMID 10499773.
- ↑ "Alpha Lipoic Acid C8H14O2S2 206.33". drugfuture.com. Retrieved 2014-07-05. Reproduction of USP32-NF27. p. 1042. Also in "Pharmacopeial Forum". 34 (5): 1209.
- ↑ Raddatz, G; Bisswanger, H (17 October 1997). "Receptor site and stereospecifity of dihydrolipoamide dehydrogenase for R- and S-lipoamide: A molecular modeling study". Journal of Biotechnology. 58 (2): 89–100. doi:10.1016/S0168-1656(97)00135-1. PMID 9383983.
- ↑ Cronan, JE; Fearnley, IM; Walker, JE (29 August 2005). "Mammalian mitochondria contain a soluble acyl carrier protein". FEBS Letters. 579 (21): 4892–6. doi:10.1016/j.febslet.2005.07.077. PMID 16109413.
- ↑ Jordan, SW; Cronan, JE, Jr. (1997). "A new metabolic link. The acyl carrier protein of lipid synthesis donates lipoic acid to the pyruvate dehydrogenase complex in Escherichia coli and mitochondria". Journal of Biological Chemistry. 272 (29): 17903–6. doi:10.1074/jbc.272.29.17903. PMID 9218413.
- ↑ Cicchillo, RM; Booker, SJ (2005). "Mechanistic investigations of lipoic acid biosynthesis in E. coli: Both sulfur atoms in lipoic acid are contributed by the same lipoyl synthase polypeptide". Journal of the American Chemical Society. 127 (9): 2860–1. doi:10.1021/ja042428u. PMID 15740115.
- ↑ Jiang, Y; Cronan, JE (2005). "Expression cloning and demonstration of Enterococcus faecalis lipoamidase (pyruvate dehydrogenase inactivase) as a Ser-Ser-Lys triad amidohydrolase". Journal of Biological Chemistry. 280 (3): 2244–56. doi:10.1074/jbc.+M408612200. PMID 15528186. Unknown parameter
|doi_brokendate=ignored (help) - ↑ Cronan, JE; Zhao, X; Jiang, Y (2005). Poole, RK, ed. "Function, attachment and synthesis of lipoic acid in Escherichia coli". Advances in microbial physiology. Advances in Microbial Physiology. 50: 103–46. doi:10.1016/S0065-2911(05)50003-1. ISBN 9780120277506. PMID 16221579.
|chapter=ignored (help) - ↑ Milne, JL; Wu, X; Borgnia, MJ; Lengyel, JS; et al. (2006). "Molecular structure of a 9-MDa icosahedral pyruvate dehydrogenase subcomplex containing the E2 and E3 enzymes using cryoelectron microscopy". Journal of Biological Chemistry. 281 (7): 4364–70. doi:10.1074/jbc.M504363200. PMC 1647297. PMID 16308322.
- ↑ Murphy, GE; Jensen, GJ (2005). "Electron cryotomography of the E. coli pyruvate and 2-oxoglutarate dehydrogenase complexes". Structure. 13 (12): 1765–73. doi:10.1016/j.str.2005.08.016. PMID 16338405.
- ↑ Machado, RS; Clark, DP; Guest, JR (1992). "Construction and properties of pyruvate dehydrogenase complexes with up to nine lipoyl domains per lipoate acetyltransferase chain". FEMS Microbiology Letters. 79 (1–3): 243–8. doi:10.1111/j.1574-6968.1992.tb14047.x. PMID 1478460.
- ↑ Douce, R; Bourguignon, J; Neuburger, M; Rebeille, F (2001). "The glycine decarboxylase system: A fascinating complex". Trends in Plant Science. 6 (4): 167–76. doi:10.1016/S1360-1385(01)01892-1. PMID 11286922.
- ↑ Durrani, AI; Schwartz, H; Nagl, M; Sontag, G (October 2010). "Determination of free [alpha]-lipoic acid in foodstuffs by HPLC coupled with CEAD and ESI-MS". Food Chemistry. 120 (4): 38329–36. doi:10.1016/j.foodchem.2009.11.045.
- ↑ Reed, LJ (October 2001). "A trail of research from lipoic acid to alpha-keto acid dehydrogenase complexes". Journal of Biological Chemistry. 276 (42): 38329–36. doi:10.1074/jbc.R100026200. PMID 11477096.
- ↑ Hermann, R; Niebch, G; Borbe, HO; Fieger, H; et al. (1996). "Enantioselective pharmacokinetics and bioavailability of different racemic formulations in healthy volunteers". European Journal of Pharmaceutical Sciences. 4 (3): 167–74. doi:10.1016/0928-0987(95)00045-3.
- ↑ Teichert, J; Preiss, R (1997). "High-performance Liquid Chromatography Methods for Determination of Lipoic and Dihydrolipoic Acid in Human Plasma". Methods in Enzymology. 279. pp. 159–66. doi:10.1016/S0076-6879(97)79019-0. ISBN 9780121821807. PMID 9211267. Missing or empty
|title=(help) - ↑ Teichert, J; Preiss, R (October 1995). "Determination of lipoic acid in human plasma by high-performance liquid chromatography with electrochemical detection". Journal of Chromatography B. 672 (2): 277–81. doi:10.1016/0378-4347(95)00225-8. PMID 8581134.
- ↑ Teichert, J; Preiss, R (November 1992). "HPLC-methods for determination of lipoic acid and its reduced form in human plasma". International Journal of Clinical Pharmacology, Therapy, and Toxicology. 30 (11): 511–2. PMID 1490813.
- ↑ Biewenga, GP; Haenen, GR; Bast, A (September 1997). "The pharmacology of the antioxidant lipoic acid". General Pharmacology. 29 (3): 315–31. doi:10.1016/S0306-3623(96)00474-0. PMID 9378235.
- ↑ Wada, M; Shigeta, Y; Inamori, K (September 1961). "A study on the metabolism of lipoic acid and lipoamide". Journal of Vitaminology. 7 (3): 237–42. doi:10.5925/jnsv1954.7.237. PMID 14004240.
- ↑ 22.0 22.1 Oizumi, J; Hayakawa, K (July 1989). "Liberation of lipoate by human serum lipoamidase from bovine heart pyruvate dehydrogenase". Biochemical and Biophysical Research Communications. 162 (2): 658–63. doi:10.1016/0006-291X(89)92361-9. PMID 2502979.
- ↑ Saito, J (1960). "[The conversion of thioctamide to thioctic acid in biological systems. I. The thioctic active substances in rabbit serum after administration of thioctamide]". Vitamin (in 日本語). 21 (3): 359–63.closed access publication – behind paywall
- ↑ Backman-Gullers, B; Hannestad, U; Nilsson, L; Sorbo, B (October 1990). "Studies on lipoamidase: Characterization of the enzyme in human serum and breast milk". Clinica Chimica Acta. 191 (1–2): 49–60. doi:10.1016/0009-8981(90)90057-Y. PMID 2127386.
- ↑ Garganta, CL; Wolf, B (August 1990). "Lipoamidase activity in human serum is due to biotinidase". Clinica Chimica Acta. 189 (3): 313–25. doi:10.1016/0009-8981(90)90313-H. PMID 2225462.
- ↑ Shih, JC; Rozo, ML; Wright, LD; McCormick, DB (February 1975). "Characterization of the growth of Pseudomonas putida LP on lipoate and its analogues: Transport, oxidation, sulphur source, and enzyme induction" (PDF). Microbiology. 86 (2): 217–27. doi:10.1099/00221287-86-2-217. PMID 1089758.
- ↑ Mansilla, MC; de Mendoza, D (February 1997). "L-cysteine biosynthesis in Bacillus subtilis: Identification, sequencing, and functional characterization of the gene coding for phosphoadenylylsulfate sulfotransferase". Journal of Bacteriology. 179 (3): 976–81. PMC 178787. PMID 9006060.
- ↑ 28.0 28.1 Schupke, H; Hempel, R; Peter, G; Hermann, R; et al. (June 2001). "New metabolic pathways of alpha-lipoic acid". Drug Metabolism and Disposition. 29 (6): 855–62. PMID 11353754.
- ↑ Teichert, J; Hermann, R; Ruus, P; Preiss, R (November 2003). "Plasma kinetics, metabolism, and urinary excretion of alpha-lipoic acid following oral administration in healthy volunteers". Journal of Clinical Pharmacology. 43 (11): 1257–67. doi:10.1177/0091270003258654. PMID 14551180.
- ↑ Hornberger, CS; Heitmiller, RF; Gunsalus, IC; Schnakenberg, GHF; et al. (1953). "Synthesis of DL—lipoic acid". Journal of the American Chemical Society. 75 (6): 1273–7. doi:10.1021/ja01102a003.
- ↑ Hornberger, CS; Heitmiller, RF; Gunsalus, IC; Schnakenberg, GHF; et al. (1952). "Synthetic preparation of lipoic acid". Journal of the American Chemical Society. 74 (9): 2382. doi:10.1021/ja01129a511.
- ↑ 32.0 32.1 Kleeman, A; Borbe, HO; Ulrich, H (1991). "Thioctic Acid-Lipoic Acid". In Borbe, HO; Ulrich, H. Thioctsäure: Neue Biochemische, Pharmakologische und Klinische Erkenntnisse zur Thioctsäure. Symposium at Wiesbaden, DE, 16-18 February 1989. Frankfurt, DE: Verlag. pp. 11–26. ISBN 9783891191255. Unknown parameter
|trans_title=ignored (help) - ↑ Fontanella, L (1955). "Preparation of optical antipodes of alpha-lipoic acid". Il Farmaco; edizione scientifica. 10 (12): 1043–5. PMID 13294188.
- ↑ Walton, E; Wagner, AF; Bachelor, FW; Peterson, LH; et al. (1955). "Synthesis of (+)-lipoic acid and its optical antipode". Journal of the American Chemical Society. 77 (19): 5144–9. Bibcode:1955JAChS..77.1678G. doi:10.1021/ja01624a057.
- ↑ Acker, DS; Wayne, WJ (1957). "Optically active and radioactive α-lipoic acids". Journal of the American Chemical Society. 79 (24): 6483. doi:10.1021/ja01581a033.
- ↑ Deguchi, Y; Miura, K (June 1964). "Studies on the synthesis of thioctic acid and its related compounds. XIV. Synthesis of (+)-thioctamide". Yakugaku Zasshi. 84: 562–3. PMID 14207116.
- ↑ Lang, G (1992). In Vitro Metabolism of a-Lipoic Acid Especially Taking Enantioselective Bio-transformation into Account (Ph.D. thesis). Münster, DE: University of Münster.
- ↑ US patent 5281722, Blaschke, G; U Scheidmantel & H Bethge et al., "Preparation and use of salts of the pure enantiomers of alpha-lipoic acid", issued 1994-01-25, assigned to DeGussa.
- ↑ 39.0 39.1 Carlson, DA; Young, KL; Fischer, SJ; Ulrich, H. "Ch. 10: An Evaluation of the Stability and Pharmacokinetics of R-lipoic Acid and R-Dihydrolipoic Acid Dosage Forms in Plasma from Healthy Human Subjects". pp. 235–70. Missing or empty
|title=(help) In Packer & Patel 2008. - ↑ Packer, L; Kraemer, K; Rimbach, G (October 2001). "Molecular aspects of lipoic acid in the prevention of diabetes complications". Nutrition. Burbank, CA. 17 (10): 888–95. doi:10.1016/S0899-9007(01)00658-X. PMID 11684397.
- ↑ 41.0 41.1 41.2 Carlson, DA; Smith, AR; Fischer, SJ; Young, KL; et al. (December 2007). "The plasma pharmacokinetics of R-(+)-lipoic acid administered as sodium R-(+)-lipoate to healthy human subjects" (PDF). Alternative Medicine Review. 12 (4): 343–51. PMID 18069903.
- ↑ Hill, AS; Werner, JA; Rogers, QR; O'Neill, SL; et al. (April 2004). "Lipoic acid is 10 times more toxic in cats than reported in humans, dogs or rats". Journal of Animal Physiology and Animal Nutrition. 88 (3–4): 150–6. doi:10.1111/j.1439-0396.2003.00472.x. PMID 15059240.
- ↑ 43.0 43.1 Packer, L; Witt, EH; Tritschler, HJ (August 1995). "Alpha-lipoic acid as a biological antioxidant". Free Radical Biology and Medicine. 19 (2): 227–50. doi:10.1016/0891-5849(95)00017-R. PMID 7649494.
- ↑ 44.0 44.1 Shay, KP; Moreau, RF; Smith, EJ; Smith, AR; et al. (October 2009). "Alpha-lipoic acid as a dietary supplement: Molecular mechanisms and therapeutic potential". Biochimica et Biophysica Acta. 1790 (10): 1149–60. doi:10.1016/j.bbagen.2009.07.026. PMC 2756298. PMID 19664690.
- ↑ Haenen, GRMM; Bast, A (1991). "Scavenging of hypochlorous acid by lipoic acid". Biochemical Pharmacology. 42 (11): 2244–6. doi:10.1016/0006-2952(91)90363-A. PMID 1659823.
- ↑ 46.0 46.1 Shay, KP; Shenvi, S; Hagen, TM. "Ch. 14 Lipoic Acid as an Inducer of Phase II Detoxification Enzymes Through Activation of Nr-f2 Dependent Gene Expression". pp. 349–71. Missing or empty
|title=(help) In Packer & Patel 2008. - ↑ Arnér, ES; Nordberg, J; Holmgren, A (August 1996). "Efficient reduction of lipoamide and lipoic acid by mammalian thioredoxin reductase". Biochemical and Biophysical Research Communications. 225 (1): 268–74. doi:10.1006/bbrc.1996.1165. PMID 8769129.
- ↑ Biaglow, JE; Ayene, IS; Koch, CJ; Donahue, J; et al. (April 2003). "Radiation response of cells during altered protein thiol redox". Radiation Research. 159 (4): 484–94. doi:10.1667/0033-7587(2003)159[0484:RROCDA]2.0.CO;2. PMID 12643793.
- ↑ Haramaki, N; Han, D; Handelman, GJ; Tritschler, HJ; et al. (1997). "Cytosolic and mitochondrial systems for NADH- and NADPH-dependent reduction of alpha-lipoic acid". Free Radical Biology and Medicine. 22 (3): 535–42. doi:10.1016/S0891-5849(96)00400-5. PMID 8981046.
- ↑ Constantinescu, A; Pick, U; Handelman, GJ; Haramaki, N; et al. (July 1995). "Reduction and transport of lipoic acid by human erythrocytes". Biochemical Pharmacology. 50 (2): 253–61. doi:10.1016/0006-2952(95)00084-D. PMID 7632170.
- ↑ May, JM; Qu, ZC; Nelson, DJ (June 2006). "Cellular disulfide-reducing capacity: An integrated measure of cell redox capacity". Biochemical and Biophysical Research Communications. 344 (4): 1352–9. doi:10.1016/j.bbrc.2006.04.065. PMID 16650819.
- ↑ Jones, W; Li, X; Qu, ZC; Perriott, L; et al. (July 2002). "Uptake, recycling, and antioxidant actions of alpha-lipoic acid in endothelial cells". Free Radical Biology and Medicine. 33 (1): 83–93. doi:10.1016/S0891-5849(02)00862-6. PMID 12086686.
- ↑ Schempp, H; Ulrich, H; Elstner, EF (1994). "Stereospecific reduction of R(+)-thioctic acid by porcine heart lipoamide dehydrogenase/diaphorase". Zeitschrift für Naturforschung C. 49 (9–10): 691–2. PMID 7945680.
- ↑ Biewenga, GP; Haenen, GRMM; Bast, A (1997). "Ch. 1: An Overview of Lipoate Chemistry". In Fuchs, J; Packer, L; Zimmer, G. Lipoic Acid In Health & Disease. CRC Press. pp. 1–32. ISBN 9780824700935.
- ↑ Lii, CK; Liu, KL; Cheng, YP; Lin, AH; et al. (May 2010). "Sulforaphane and alpha-lipoic acid upregulate the expression of the pi class of glutathione S-transferase through c-jun and Nrf2 activation". Journal of Nutrition. 140 (5): 885–92. doi:10.3945/jn.110.121418. PMID 20237067.
- ↑ Gal, EM; Razevska, DE (August 1960). "Studies on the in vivo metabolism of lipoic acid. 1. The fate of DL-lipoic acid-S35 in normal and thiamine-deficient rats". Archives of Biochemistry and Biophysics. 89 (2): 253–61. doi:10.1016/0003-9861(60)90051-5. PMID 13825981.
- ↑ Gal, EM (July 1965). "Reversal of selective toxicity of (-)-alpha-lipoic acid by thiamine in thiamine-deficient rats". Nature. 207 (996): 535. Bibcode:1965Natur.207..535G. doi:10.1038/207535a0. PMID 5328673.
- ↑ US patent 6271254, Ulrich, H; CH Weischer & J Engel et al., "Pharmaceutical compositions containing R-alpha-lipoic acid or S-alpha.-lipoic acid as active ingredient", issued 2001-08-07, assigned to ASTA Pharma.
- ↑ Kilic, F; Handelman, GJ; Serbinova, E; Packer, L; et al. (October 1995). "Modelling cortical cataractogenesis 17: In vitro effect of a-lipoic acid on glucose-induced lens membrane damage, a model of diabetic cataractogenesis". Biochemistry and Molecular Biology International. 37 (2): 361–70. PMID 8673020.
- ↑ Artwohl, M; Schmetterer, L; Rainer, G; et al. (September 2000). "Modulation by antioxidants of endothelial apoptosis, proliferation, & associated gene/protein expression". 36th Annual Meeting of the European Association for the Study of Diabetes, 17-21 September 2000, Jerusalem, Israel. Diabetologia. 43 (Suppl 1) (published August 2000). Abs 274. PMID 11008622. Missing or empty
|title=(help) - ↑ Streeper, RS; Henriksen, EJ; Jacob, S; Hokama, JY; et al. (July 1997). "Differential effects of lipoic acid stereoisomers on glucose metabolism in insulin-resistant skeletal muscle". AJP - Endocrinology and Metabolism. 273 (1 Pt 1): E185–91. PMID 9252495.
- ↑ Frölich, L; Götz, ME; Weinmüller, M; Youdim, MB; et al. (March 2004). "(r)-, but not (s)-alpha lipoic acid stimulates deficient brain pyruvate dehydrogenase complex in vascular dementia, but not in Alzheimer dementia". Journal of Neural Transmission. 111 (3): 295–310. doi:10.1007/s00702-003-0043-5. PMID 14991456.
- ↑ Ziegle, D.; Reljanovic, M; Mehnert, H; Gries, F. A. (1999). "α-Lipoic acid in the treatment of diabetic polyneuropathy in Germany". Experimental and Clinical Endocrinology & Diabetes. J. A. Barth Verlag in Georg Thieme Verlag KG Stuttgart. 107 (7): 421–30. doi:10.1055/s-0029-1212132. PMID 10595592. Retrieved 28 August 2014.
- ↑ "Lipoic Acid". American Cancer Society. November 2008. Retrieved 5 October 2013.
- ↑ Mijnhout GS, Kollen BJ, Alkhalaf A, Kleefstra N, Bilo HJ; Kollen; Alkhalaf; Kleefstra; Bilo (2012). "Alpha lipoic Acid for symptomatic peripheral neuropathy in patients with diabetes: a meta-analysis of randomized controlled trials". Int J Endocrinol (Meta-analysis). 2012: 456279. doi:10.1155/2012/456279. PMC 3272801. PMID 22331979.
- ↑ Sauer J, Tabet N, Howard R; Tabet; Howard; Howard (2008). "Alpha lipoic acid for dementia". Cochrane Database Syst Rev (Systematic review) (1): CD004244. doi:10.1002/14651858.CD004244.pub2. PMID 14974062.
- ↑ Patton LL, Siegel MA, Benoliel R, De Laat A; Siegel; Benoliel; De Laat (March 2007). "Management of burning mouth syndrome: systematic review and management recommendations". Oral Surg Oral Med Oral Pathol Oral Radiol Endod (Systematic review). 103 Suppl: S39.e1–13. doi:10.1016/j.tripleo.2006.11.009. PMID 17379153.
- ↑ Pfeffer G, Majamaa K, Turnbull DM, Thorburn D, Chinnery PF; Majamaa; Turnbull; Thorburn; Chinnery (2012). "Treatment for mitochondrial disorders". Cochrane Database Syst Rev (Systematic review). 4: CD004426. doi:10.1002/14651858.CD004426.pub3. PMID 22513923.
- ↑ Salinthone S, Yadav V, Bourdette DN, Carr DW; Yadav; Bourdette; Carr (June 2008). "Lipoic acid: a novel therapeutic approach for multiple sclerosis and other chronic inflammatory diseases of the CNS". Endocr Metab Immune Disord Drug Targets (Report). 8 (2): 132–42. doi:10.2174/187153008784534303. PMID 18537699.
Based on EAE studies and the preliminary results from this clinical trial, LA appears to have a potential of a useful drug in treating MS.
- ↑ Ishida, Y; Ohara, T; Okuno, Y; Ito, T; et al. (2007). "Alpha-lipoic acid and insulin autoimmune syndrome". Diabetes Care. 30 (9): 2240–1. doi:10.2337/dc07-0689. PMID 17586737.
- Packer, Lester; Patel, Mulchand S., eds. (2008). Lipoic Acid: Energy Production, Antioxidant Activity and Health Effects. Boca Raton, FL: CRC Press. ISBN 1420045377.
Further reading
- Pages with script errors
- Pages with citations using unsupported parameters
- CS1 errors: chapter ignored
- Pages with citations lacking titles
- CS1 日本語-language sources (ja)
- Pages with broken file links
- CS1 maint: Multiple names: authors list
- Chemical articles with multiple compound IDs
- Multiple chemicals in an infobox that need indexing
- Chemical articles with multiple CAS registry numbers
- Articles without UNII source
- Chemical articles with unknown parameter in Chembox
- ECHA InfoCard ID from Wikidata
- Articles containing unverified chemical infoboxes
- All articles with unsourced statements
- Articles with unsourced statements from April 2014
- Articles with invalid date parameter in template
- Antioxidants
- Carboxylic acids
- Cofactors
- Eli Lilly and Company
- Organic disulfides
- Dithiolanes