Intravascular device related infections
| Intravascular device related infections |
Intravascular catheters are indispensable in modern-day medical practice, particularly in intensive care units (ICUs). Although such catheters provide necessary vascular access, their use puts patients at risk for local and systemic infectious complications, including local site infection, catheter-related bloodstream infections (CRBSI), septic thrombophlebitis, endocarditis, and other metastatic infections (e.g., lung abscess, brain abscess, osteomyelitis, and endophthalmitis).
Health-care institutions purchase millions of intravascular catheters each year. The incidence of CRBSI varies considerably by type of catheter, frequency of catheter manipulation, and patient-related factors (e.g., underlying disease and acuity of illness). Peripheral venous catheters are the devices most frequently used for vascular access. Although the incidence of local or bloodstream infections (BSIs) associated with peripheral venous catheters is usually low, serious infectious complications produce considerable annual morbidity because of the frequency with which such catheters are used. However, the majority of serious catheter-related infections are associated with central venous catheters (CVCs), especially those that are placed in patients in ICUs.
In the ICU setting, the incidence of infection is often higher than in the less acute in-patient or ambulatory setting. In the ICU, central venous access might be needed for extended periods of time; patients can be colonized with hospital-acquired organisms; and the catheter can be manipulated multiple times per day for the administration of fluids, drugs, and blood products. Moreover, some catheters can be inserted in urgent situations, during which optimal attention to aseptic technique might not be feasible. Certain catheters (e.g., pulmonary artery catheters and peripheral arterial catheters) can be accessed multiple times per day for hemodynamic measurements or to obtain samples for laboratory analysis, augmenting the potential for contamination and subsequent clinical infection.
The magnitude of the potential for CVCs to cause morbidity and mortality resulting from infectious complications has been estimated in several studies [1]. In the United States, 15 million CVC days (i.e., the total number of days of exposure to CVCs by all patients in the selected population during the selected time period) occur in ICUs each year [2]. If the average rate of CVC-associated BSIs is 5.3 per 1,000 catheter days in the ICU [3], approximately 80,000 CVC-associated BSIs occur in ICUs each year in the United States. The attributable mortality for these BSIs has ranged from no increase in mortality in studies that controlled for severity of illness ([4] [5] [6]), to 35% increase in mortality in prospective studies that did not use this control ([7] [8]). Thus, the attributable mortality remains unclear. The attributable cost per infection is an estimated $34,508–$56,000 ([9] [10]), and the annual cost of caring for patients with CVC-associated BSIs ranges from $296 million to $2.3 billion [11].
A total of 250,000 cases of CVC-associated BSIs have been estimated to occur annually if entire hospitals are assessed rather than ICUs exclusively [12]. In this case, attributable mortality is an estimated 12%–25% for each infection, and the marginal cost to the health-care system is $25,000 per episode [13]. Therefore, by several analyses, the cost of CVC-associated BSI is substantial, both in terms of morbidity and in terms of financial resources expended. To improve patient outcome and reduce health-care costs, strategies should be implemented to reduce the incidence of these infections. This effort should be multidisciplinary, involving health-care professionals who insert and maintain intravascular catheters, health-care managers who allocate resources, and patients who are capable of assisting in the care of their catheters. Although several individual strategies have been studied and shown to be effective in reducing CRBSI, studies using multiple strategies have not been conducted. Thus, it is not known whether implementing multiple strategies will have an additive effect in reducing CRBSI, but it is logical to use multiple strategies concomitantly.
Terminology and Estimates of Risk
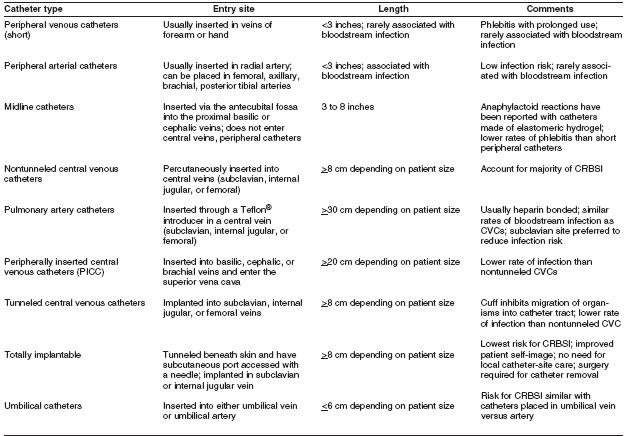
The terminology used to identify different types of catheters is confusing, because many clinicians and researchers use different aspects of the catheter for informal reference. A catheter can be designated by the type of vessel it occupies (e.g., peripheral venous, central venous, or arterial); its intended life span (e.g., temporary or short-term versus permanent or long-term); its site of insertion (e.g., subclavian, femoral, internal jugular, peripheral, and peripherally inserted central catheter [PICC]); its pathway from skin to vessel (e.g., tunneled versus nontunneled); its physical length (e.g., long versus short); or some special characteristic of the catheter (e.g., presence or absence of a cuff, impregnation with heparin, antibiotics or antiseptics, and the number of lumens). To accurately define a specific type of catheter, all of these aspects should be described.
The rate of all catheter-related infections (including local infections and systemic infections) is difficult to determine. Although CRBSI is an ideal parameter because it represents the most serious form of catheter-related infection, the rate of such infection depends on how CRBSI is defined.
Health-care professionals should recognize the difference between surveillance definitions and clinical definitions. The surveillance definitions for catheter-associated BSI includes all BSIs that occur in patients with CVCs, when other sites of infection have been excluded (Appendix A). That is, the surveillance definition overestimates the true incidence of CRBSI because not all BSIs originate from a catheter. Some bacteremias are secondary BSIs from undocumented sources (e.g., postoperative surgical sites, intra-abdominal infections, and hospital-associated pneumonia or urinary tract infections).
Thus, surveillance definitions are really definitions for catheter-associated BSIs. A more rigorous definition might include only those BSIs for which other sources were excluded by careful examination of the patient record, and where a culture of the catheter tip demonstrated substantial colonies of an organism identical to those found in the bloodstream. Such a clinical definition would focus on catheter-related BSIs. Therefore, to accurately compare a health-care facility’s infection rate to published data, comparable definitions also should be used.
CDC and the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) recommend that the rate of catheter-associated BSIs be expressed as the number of catheter associated BSIs per 1,000 CVC days ([14] [15]). This parameter is more useful than the rate expressed as the number of catheter-associated infections per 100 catheters (or percentage of catheters studied), because it accounts for BSIs over time and therefore adjusts risk for the number of days the catheter is in use.
Epidemiology and Microbiology
Since 1970, CDC’s National Nosocomial Infection Surveillance System (NNIS) has been collecting data on the incidence and etiologies of hospital-acquired infections, including CVC-associated BSIs in a group of nearly 300 U.S. hospitals. The majority of hospital-acquired BSIs are associated with the use of a CVC, with BSI rates being substantially higher among patients with CVCs than among those without CVCs. Rates of CVC-associated BSI vary considerably by hospital size, hospital service/unit, and type of CVC.
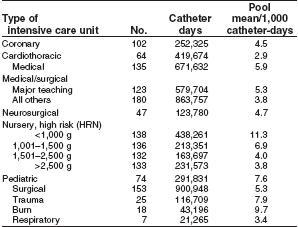
During 1992–2001, NNIS hospitals reported ICU rates of CVC-associated BSI ranging from 2.9 (in a cardiothoracic ICU) to 11.3 (in a neonatal nursery for infants weighing <1,000 g) BSIs per 1,000 CVC days [16].
The relative risk of catheter-associated BSI also has been assessed in a meta-analysis of 223 prospective studies of adult patients (11). Relative risk of infection was best determined by analyzing rates of infection both by BSIs per 100 catheters and BSIs per 1,000 catheter days. These rates, and the NNIS derived data, can be used as benchmarks by individual hospitals to estimate how their rates compare with other institutions.
Rates are influenced by patient-related parameters, such as severity of illness and type of illness (e.g., third-degree burns versus postcardiac surgery), and by catheter-related parameters, such as the condition under which the catheter was placed (e.g., elective versus urgent) and catheter type (e.g., tunneled versus nontunneled or subclavian versus jugular).
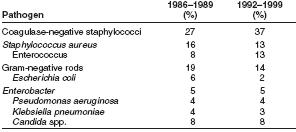
Types of organisms that most commonly cause hospital acquired BSIs change over time. During 1986–1989, coagulase negative staphylococci, followed by Staphylococcus aureus, were the most frequently reported causes of BSIs, accounting for 27% and 16% of BSIs, respectively [17]
Pooled data from 1992 through 1999 indicate that coagulase negative staphylococci, followed by enterococci, are now the most frequently isolated causes of hospital-acquired BSIs. [18]
Coagulase-negative staphylococci account for 37% (12) and S. aureus account for 12.6% of reported hospital-acquired BSIs (12). Also notable was the susceptibility pattern of S. aureus isolates. In 1999, for the first time since NNIS has been reporting susceptibilities, >50% of all S. aureus isolates from ICUs were resistant to oxacillin. [19]
In 1999, enterococci accounted for 13.5% of BSIs, an increase from 8% reported to NNIS during 1986–1989. The percentage of enterococcal ICU isolates resistant to vancomycin also is increasing, escalating from 0.5% in 1989 to 25.9% in 1999 [20].
Candida spp. caused 8% of hospital-acquired BSIs reported to NNIS during 1986–1989 (15,16), and during 1992–1999. [21] [22][23] Resistance of Candida spp. to commonly used