Intravascular device related infections: Difference between revisions
No edit summary |
No edit summary |
||
| Line 28: | Line 28: | ||
==Terminology and Estimates of Risk== | ==Terminology and Estimates of Risk== | ||
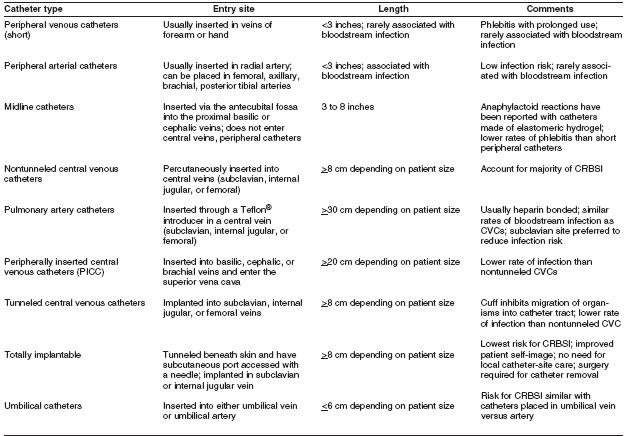
The terminology used to identify different types of catheters is confusing, because many clinicians and researchers use different aspects of the catheter for informal reference. A catheter can be designated by the type of vessel it occupies (e.g., peripheral venous, central venous, or arterial); its intended life span (e.g., temporary or short-term versus permanent or long-term); its site of insertion (e.g., subclavian, femoral, internal jugular, peripheral, and peripherally inserted central catheter [PICC]); its pathway from skin to vessel (e.g., tunneled versus nontunneled); its physical length (e.g., long versus short); or some special characteristic of the catheter (e.g., presence or absence of a cuff, impregnation with heparin, antibiotics or antiseptics, and the number of lumens). To | The terminology used to identify different types of catheters is confusing, because many clinicians and researchers use different aspects of the catheter for informal reference. A catheter can be designated by the type of vessel it occupies (e.g., peripheral venous, central venous, or arterial); its intended life span (e.g., temporary or short-term versus permanent or long-term); its site of insertion (e.g., subclavian, femoral, internal jugular, peripheral, and peripherally inserted central catheter [PICC]); its pathway from skin to vessel (e.g., tunneled versus nontunneled); its physical length (e.g., long versus short); or some special characteristic of the catheter (e.g., presence or absence of a cuff, impregnation with heparin, antibiotics or antiseptics, and the number of lumens). To accurately define a specific type of catheter, all of these aspects should be described. | ||
[[Image:IVC Table 1.jpg|Center|800px|Table 1]] | |||
The rate of all catheter-related infections (including local infections and systemic infections) is difficult to determine. Although CRBSI is an ideal parameter because it represents the most serious form of catheter-related infection, the rate of such infection depends on how CRBSI is defined. | |||
Health-care professionals should recognize the difference between surveillance definitions and clinical definitions. The surveillance definitions for catheter-associated BSI includes all BSIs that occur in patients with CVCs, when other sites of infection have been excluded (Appendix A). That is, the surveillance definition overestimates the true incidence of CRBSI because not all BSIs originate from a catheter. Some bacteremias are secondary BSIs from undocumented sources (e.g., postoperative surgical sites, intra-abdominal infections, and hospital-associated pneumonia or urinary tract infections). | |||
Thus, surveillance definitions are really definitions for | |||
Revision as of 21:48, 7 January 2009
| Intravascular device related infections |
Intravascular catheters are indispensable in modern-day medical practice, particularly in intensive care units (ICUs). Although such catheters provide necessary vascular access, their use puts patients at risk for local and systemic infectious complications, including local site infection, catheter-related bloodstream infections (CRBSI), septic thrombophlebitis, endocarditis, and other metastatic infections (e.g., lung abscess, brain abscess, osteomyelitis, and endophthalmitis).
Health-care institutions purchase millions of intravascular catheters each year. The incidence of CRBSI varies considerably by type of catheter, frequency of catheter manipulation, and patient-related factors (e.g., underlying disease and acuity of illness). Peripheral venous catheters are the devices most frequently used for vascular access. Although the incidence of local or bloodstream infections (BSIs) associated with peripheral venous catheters is usually low, serious infectious complications produce considerable annual morbidity because of the frequency with which such catheters are used. However, the majority of serious catheter-related infections are associated with central venous catheters (CVCs), especially those that are placed in patients in ICUs.
In the ICU setting, the incidence of infection is often higher than in the less acute in-patient or ambulatory setting. In the ICU, central venous access might be needed for extended periods of time; patients can be colonized with hospital-acquired organisms; and the catheter can be manipulated multiple times per day for the administration of fluids, drugs, and blood products. Moreover, some catheters can be inserted in urgent situations, during which optimal attention to aseptic technique might not be feasible. Certain catheters (e.g., pulmonary artery catheters and peripheral arterial catheters) can be accessed multiple times per day for hemodynamic measurements or to obtain samples for laboratory analysis, augmenting the potential for contamination and subsequent clinical infection.
The magnitude of the potential for CVCs to cause morbidity and mortality resulting from infectious complications has been estimated in several studies [1]. In the United States, 15 million CVC days (i.e., the total number of days of exposure to CVCs by all patients in the selected population during the selected time period) occur in ICUs each year [2]. If the average rate of CVC-associated BSIs is 5.3 per 1,000 catheter days in the ICU [3], approximately 80,000 CVC-associated BSIs occur in ICUs each year in the United States. The attributable mortality for these BSIs has ranged from no increase in mortality in studies that controlled for severity of illness ([4] [5] [6]), to 35% increase in mortality in prospective studies that did not use this control ([7] [8]). Thus, the attributable mortality remains unclear. The attributable cost per infection is an estimated $34,508–$56,000 ([9] [10]), and the annual cost of caring for patients with CVC-associated BSIs ranges from $296 million to $2.3 billion [11].
A total of 250,000 cases of CVC-associated BSIs have been estimated to occur annually if entire hospitals are assessed rather than ICUs exclusively [12]. In this case, attributable mortality is an estimated 12%–25% for each infection, and the marginal cost to the health-care system is $25,000 per episode [13]. Therefore, by several analyses, the cost of CVC-associated BSI is substantial, both in terms of morbidity and in terms of financial resources expended. To improve patient outcome and reduce health-care costs, strategies should be implemented to reduce the incidence of these infections. This effort should be multidisciplinary, involving health-care professionals who insert and maintain intravascular catheters, health-care managers who allocate resources, and patients who are capable of assisting in the care of their catheters. Although several individual strategies have been studied and shown to be effective in reducing CRBSI, studies using multiple strategies have not been conducted. Thus, it is not known whether implementing multiple strategies will have an additive effect in reducing CRBSI, but it is logical to use multiple strategies concomitantly.
Terminology and Estimates of Risk
The terminology used to identify different types of catheters is confusing, because many clinicians and researchers use different aspects of the catheter for informal reference. A catheter can be designated by the type of vessel it occupies (e.g., peripheral venous, central venous, or arterial); its intended life span (e.g., temporary or short-term versus permanent or long-term); its site of insertion (e.g., subclavian, femoral, internal jugular, peripheral, and peripherally inserted central catheter [PICC]); its pathway from skin to vessel (e.g., tunneled versus nontunneled); its physical length (e.g., long versus short); or some special characteristic of the catheter (e.g., presence or absence of a cuff, impregnation with heparin, antibiotics or antiseptics, and the number of lumens). To accurately define a specific type of catheter, all of these aspects should be described.
The rate of all catheter-related infections (including local infections and systemic infections) is difficult to determine. Although CRBSI is an ideal parameter because it represents the most serious form of catheter-related infection, the rate of such infection depends on how CRBSI is defined.
Health-care professionals should recognize the difference between surveillance definitions and clinical definitions. The surveillance definitions for catheter-associated BSI includes all BSIs that occur in patients with CVCs, when other sites of infection have been excluded (Appendix A). That is, the surveillance definition overestimates the true incidence of CRBSI because not all BSIs originate from a catheter. Some bacteremias are secondary BSIs from undocumented sources (e.g., postoperative surgical sites, intra-abdominal infections, and hospital-associated pneumonia or urinary tract infections).
Thus, surveillance definitions are really definitions for