Hepatic cysts: Difference between revisions
No edit summary |
No edit summary |
||
| Line 46: | Line 46: | ||
==Bile Duct Hamartomas== | ==Bile Duct Hamartomas== | ||
Bile duct hamartomas (BDH) – also referred to as ‘simple cysts’ and ‘von-meyenberg complexes’ – are uncommon but not rare. They are benign and do not transform into malignancies. Small cysts can be found in as many as 7.3% of people referred for abdominal MRI while giant, symptomatic cysts are present in 0.4% of the population referred for such imaging.<ref> Tapper EB, Adsay NV, Kalb B, Martin D, Kooby D, Sarmiento JM. Symptomatic Bile Duct Hamartomas: Surgical Management in an MRI Driven Practice. | Bile duct hamartomas (BDH) – also referred to as ‘simple cysts’ and ‘von-meyenberg complexes’ – are uncommon but not rare. They are benign and do not transform into malignancies. Small cysts can be found in as many as 7.3% of people referred for abdominal MRI while giant, symptomatic cysts are present in 0.4% of the population referred for such imaging.<ref> Tapper EB, Adsay NV, Kalb B, Martin D, Kooby D, Sarmiento JM. Symptomatic Bile Duct Hamartomas: Surgical Management in an MRI Driven Practice. J Gastrointest Surg. 2010 May 18. [Epub ahead of print]</ref> BDH are more common in women, in a ratio of 4:1 or more, and the average age of presentation is 60 years old. <ref> Regev A et al. Large Cystic Lesions of the Liver in Adults:A 15-Year Experience in a Tertiary Center. J Am Coll Surg 2001;193:36–45</ref><ref> Gamblin TC, Holloway SE, Heckman JT, Geller DA. Laparoscopic resection of benign hepatic cysts: a new standard. J Am Coll Surg. Nov 2008;207(5):731-736.</ref> Pain is, by far, the most common presentation, but patients have also complained of jaundice, edema and have presented, rarely, with a ruptured cyst. | ||
===Diagnosis=== | ===Diagnosis=== | ||
| Line 52: | Line 52: | ||
The diagnosis of BDH pre-operatively is important. Lab tests typically do not yield any information. In general, patients are diagnosed by ultrasound, after which they receive abdominal CT .<ref> Regev A et al. Large Cystic Lesions of the Liver in Adults:A 15-Year Experience in a Tertiary Center. J Am Coll Surg 2001;193:36–45</ref> Problematically, it is impossible to distinguish BDH from cystadenomas by CT scan, and cystadenomas can degenerate into carcinoma. Up to 10% of simple cysts evaluated by CT turn out to be cystadenomas on final pathology. <ref>Gamblin TC, Holloway SE, Heckman JT, Geller DA. Laparoscopic resection of benign hepatic cysts: a new standard. J Am Coll Surg. Nov 2008;207(5):731-736.</ref> Therefore, depending on the type of surgery received, this may mean a lifetime of follow-up scans in surveillance of recurrence and cancer. | The diagnosis of BDH pre-operatively is important. Lab tests typically do not yield any information. In general, patients are diagnosed by ultrasound, after which they receive abdominal CT .<ref> Regev A et al. Large Cystic Lesions of the Liver in Adults:A 15-Year Experience in a Tertiary Center. J Am Coll Surg 2001;193:36–45</ref> Problematically, it is impossible to distinguish BDH from cystadenomas by CT scan, and cystadenomas can degenerate into carcinoma. Up to 10% of simple cysts evaluated by CT turn out to be cystadenomas on final pathology. <ref>Gamblin TC, Holloway SE, Heckman JT, Geller DA. Laparoscopic resection of benign hepatic cysts: a new standard. J Am Coll Surg. Nov 2008;207(5):731-736.</ref> Therefore, depending on the type of surgery received, this may mean a lifetime of follow-up scans in surveillance of recurrence and cancer. | ||
There is mounting evidence that MRI can be used to pre-operatively differentiates BDH from cystadenomas.<ref>Semelka RC, Hussain SM, Marcos HB, Woosley JT. Biliary hamartomas: solitary and multiple lesions shown on current MR techniques including gadolinium enhancement. J Magn Reson Imaging. 1999;10(2):196-201.</ref><ref>Tohme-Noun C, Cazals D, Noun R, Menassa L, Valla D, Vilgrain V. Multiple biliary hamartomas: magnetic resonance features with histopathologic correlation. Eur Radiol. Mar 2008;18(3):493-499.</ref><ref>Zheng RQ, Zhang B, Kudo M, Onda H, Inoue T. Imaging findings of biliary hamartomas. World J Gastroenterol 2005; 11:6354-6359.</ref><ref> Tapper EB, Adsay NV, Kalb B, Martin D, Kooby D, Sarmiento JM. Symptomatic Bile Duct Hamartomas: Surgical Management in an MRI Driven Practice. Accepted by Journal of Gastrointestinal Surgery (Feb 2010)</ref> On MRI, BDH have well-defined, lobulated margins, thin septations, a thin rim of peripheral enhancement on T2 weighted images, internal fluid content that may contain hemorrhage and/or proteinaceaous material and are without any intracystic vascularized soft tissue elements.<ref> | There is mounting evidence that MRI can be used to pre-operatively differentiates BDH from cystadenomas.<ref>Semelka RC, Hussain SM, Marcos HB, Woosley JT. Biliary hamartomas: solitary and multiple lesions shown on current MR techniques including gadolinium enhancement. J Magn Reson Imaging. 1999;10(2):196-201.</ref><ref>Tohme-Noun C, Cazals D, Noun R, Menassa L, Valla D, Vilgrain V. Multiple biliary hamartomas: magnetic resonance features with histopathologic correlation. Eur Radiol. Mar 2008;18(3):493-499.</ref><ref>Zheng RQ, Zhang B, Kudo M, Onda H, Inoue T. Imaging findings of biliary hamartomas. World J Gastroenterol 2005; 11:6354-6359.</ref><ref> Tapper EB, Adsay NV, Kalb B, Martin D, Kooby D, Sarmiento JM. Symptomatic Bile Duct Hamartomas: Surgical Management in an MRI Driven Practice. Accepted by Journal of Gastrointestinal Surgery (Feb 2010)</ref> On MRI, BDH have well-defined, lobulated margins, thin septations, a thin rim of peripheral enhancement on T2 weighted images, internal fluid content that may contain hemorrhage and/or proteinaceaous material and are without any intracystic vascularized soft tissue elements.<ref> apper EB, Adsay NV, Kalb B, Martin D, Kooby D, Sarmiento JM. Symptomatic Bile Duct Hamartomas: Surgical Management in an MRI Driven Practice. J Gastrointest Surg. 2010 May 18. [Epub ahead of print]</ref> By contrast, MRI evaluation of biliary cystadenomas and cystadenocarcinomas show smooth margins, mural nodules, and papillary projections without an enhancing peripheral rim.<ref>Lewin M, Mourra N, Honigman I, et al. Assessment of MRI and MRCP in diagnosis of biliary cystadenoma and cystadenocarcinoma. Eur Radiol 2006;16(2):407-413</ref> | ||
===Management=== | ===Management=== | ||
The management of BDH has two principal considerations. First, if the patient is asymptomatic, it reasonable to observe the patient and not intervene. Second, if there a confident diagnosis that precludes the possibility of a cystadenoma (which some researchers argue can be achieved using MRI) and patient requires an intervention, the consensus is that the operation of choice is a laparoscopic fenestration. This is usually a brief procedure that involves ‘unroofing’ the cyst with minimal – in any – hepatic resection.<ref>Katkhouda A et al. Laparoscopic Management of Benign Solid and Cystic Lesions of the Liver. Annals of Surgery 1999; 229(4):460-466.</ref><ref>Gamblin TC, Holloway SE, Heckman JT, Geller DA. Laparoscopic resection of benign hepatic cysts: a new standard. J Am Coll Surg. Nov 2008;207(5):731-736.</ref><ref> | The management of BDH has two principal considerations. First, if the patient is asymptomatic, it reasonable to observe the patient and not intervene. Second, if there a confident diagnosis that precludes the possibility of a cystadenoma (which some researchers argue can be achieved using MRI) and patient requires an intervention, the consensus is that the operation of choice is a laparoscopic fenestration. This is usually a brief procedure that involves ‘unroofing’ the cyst with minimal – in any – hepatic resection.<ref>Katkhouda A et al. Laparoscopic Management of Benign Solid and Cystic Lesions of the Liver. Annals of Surgery 1999; 229(4):460-466.</ref><ref>Gamblin TC, Holloway SE, Heckman JT, Geller DA. Laparoscopic resection of benign hepatic cysts: a new standard. J Am Coll Surg. Nov 2008;207(5):731-736.</ref><ref>apper EB, Adsay NV, Kalb B, Martin D, Kooby D, Sarmiento JM. Symptomatic Bile Duct Hamartomas: Surgical Management in an MRI Driven Practice. J Gastrointest Surg. 2010 May 18. [Epub ahead of print]</ref> These cysts should not be drained percutaneously as they will only reccur and instrumentation can lead to infection. | ||
==Cystadenoma and Cystadenocarcinomas== | ==Cystadenoma and Cystadenocarcinomas== | ||
Revision as of 01:22, 23 May 2010
| Hepatic cysts | |
 | |
|---|---|
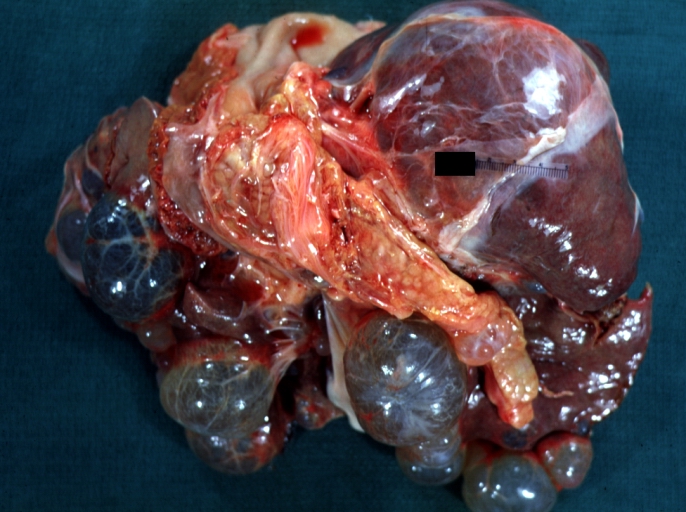
| Liver: Multiple Cysts: Gross natural color external view of liver Image courtesy of Professor Peter Anderson DVM PhD and published with permission © PEIR, University of Alabama at Birmingham, Department of Pathology |
|
WikiDoc Resources for Hepatic cysts |
|
Articles |
|---|
|
Most recent articles on Hepatic cysts Most cited articles on Hepatic cysts |
|
Media |
|
Powerpoint slides on Hepatic cysts |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Hepatic cysts at Clinical Trials.gov Trial results on Hepatic cysts Clinical Trials on Hepatic cysts at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Hepatic cysts NICE Guidance on Hepatic cysts
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Hepatic cysts Discussion groups on Hepatic cysts Patient Handouts on Hepatic cysts Directions to Hospitals Treating Hepatic cysts Risk calculators and risk factors for Hepatic cysts
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Hepatic cysts |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-in-Chief: Elliot B. Tapper, MD. Department of Medicine. Beth Israel Deaconess Medical Center
Associate Editors-in-Chief: C. Michael Gibson, M.S., M.D., Cafer Zorkun, M.D.
Overview
Liver cysts are common, detected in as much as 4-7% of the general population,[1][2] and are the result of many causes. Most cysts are found incidentally, on imaging studies ordered for other reasons altogether. Some cysts, however, can become symptomatic – causing pain, nausea, jaundice, compressing other abdominal structures, rupturing or compromising the function of the liver.[3] When symptomatic, an intervention is often indicated. As the causes are so diverse, it will be useful to discuss these cysts, their diagnosis and their management, separately.
Differential Diagnosis
- Aerobic gram negative bacteria
- Amebiasis
- Amebic cyst
- Bile duct hamartoma
- Clostridium Difficile
- Cystadenoma and Cystadenocarcinomas
- Echinococcal cyst
- Hydatid Cyst
- Inflammatory cyst
- Neisseria gonorrhea
- Neoplastic cyst
- Peliosis hepatitis
- Polycystic liver disease
- Retention cyst
- Solitary cyst
- Staphylococcal Infections
- Streptococcal Infections
- Syphilis
Fibropolycystic Disease
Most commonly, liver cysts are the result of malformed and dilated bile ducts, set in the background of fibrous stroma, thought to be constituents of the spectrum of fibropolycystic disease including congenital hepatic fibrosis, autosomal recessive polycystic kidney disease, bile duct atresia, Caroli’s disease, and mesenchymal hamartomas.[4] While the understanding of these lesions is evolving, there are three major categories: bile duct hamartomas, cystadenomas and cystedocarcinomas.
Bile Duct Hamartomas
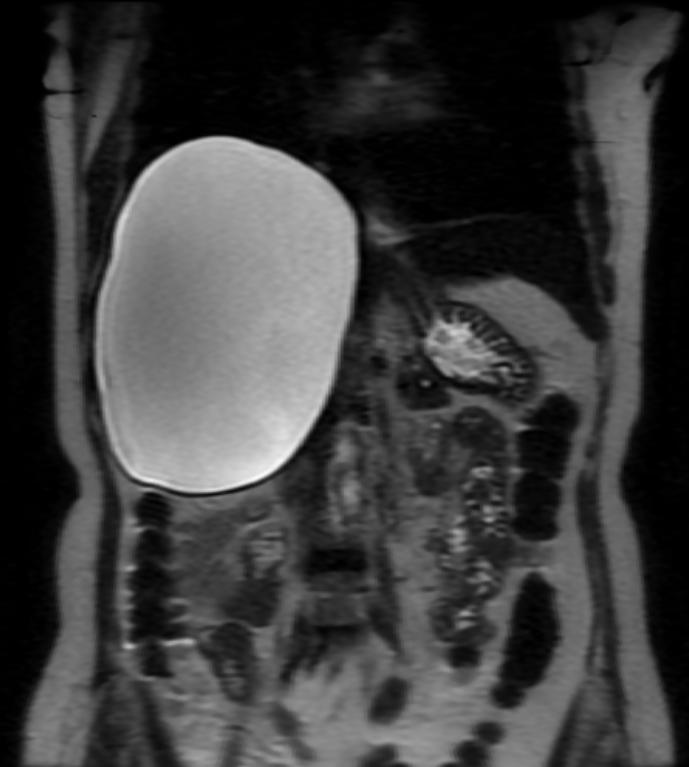
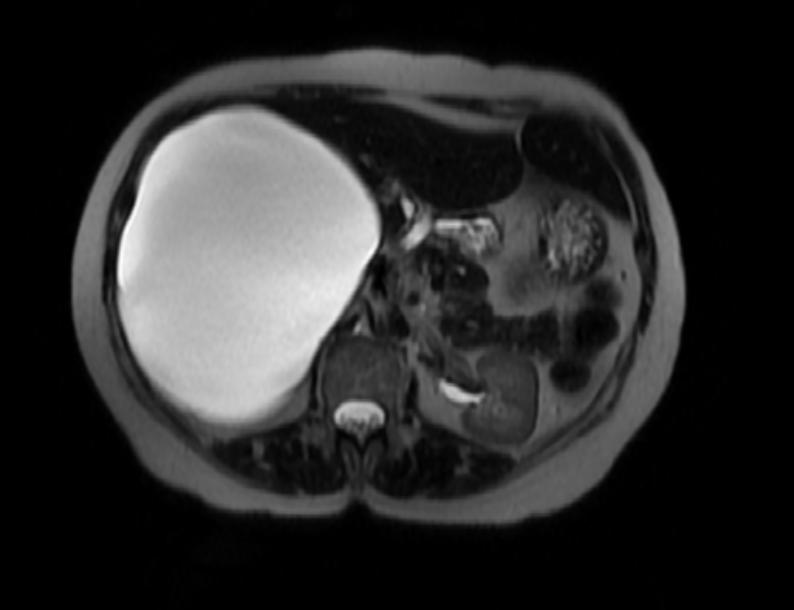
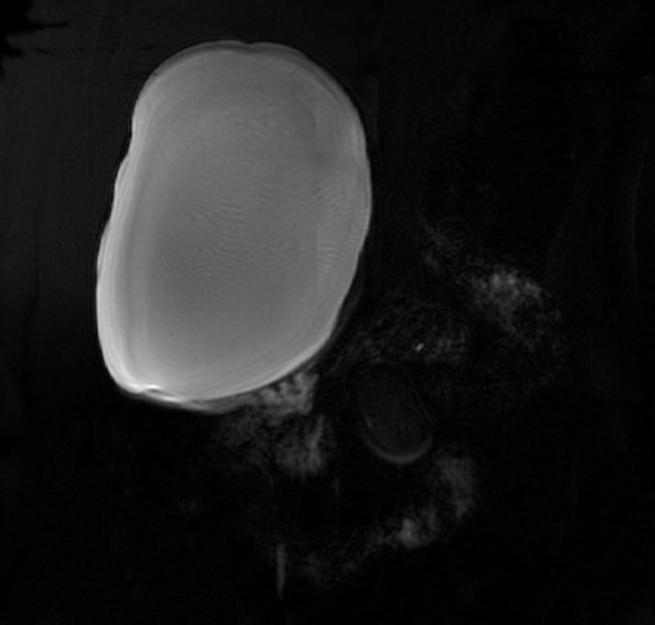
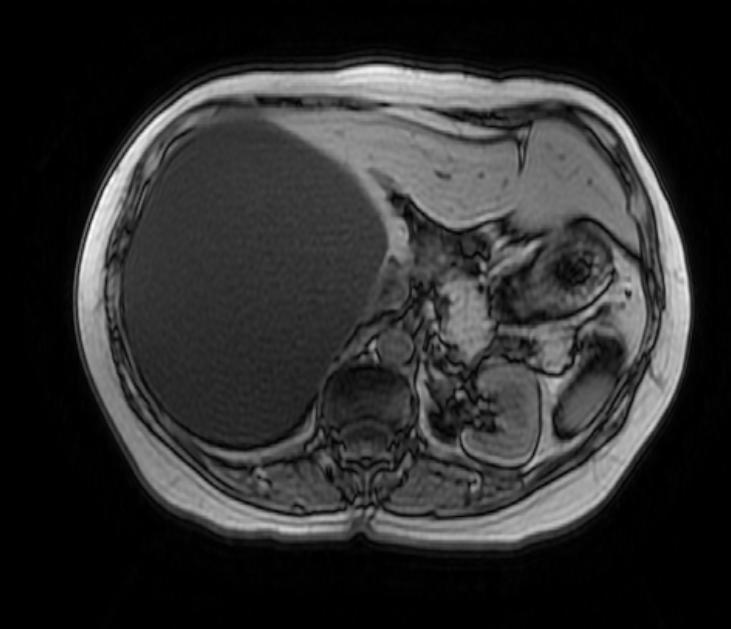
Bile duct hamartomas (BDH) – also referred to as ‘simple cysts’ and ‘von-meyenberg complexes’ – are uncommon but not rare. They are benign and do not transform into malignancies. Small cysts can be found in as many as 7.3% of people referred for abdominal MRI while giant, symptomatic cysts are present in 0.4% of the population referred for such imaging.[5] BDH are more common in women, in a ratio of 4:1 or more, and the average age of presentation is 60 years old. [6][7] Pain is, by far, the most common presentation, but patients have also complained of jaundice, edema and have presented, rarely, with a ruptured cyst.
Diagnosis
The diagnosis of BDH pre-operatively is important. Lab tests typically do not yield any information. In general, patients are diagnosed by ultrasound, after which they receive abdominal CT .[8] Problematically, it is impossible to distinguish BDH from cystadenomas by CT scan, and cystadenomas can degenerate into carcinoma. Up to 10% of simple cysts evaluated by CT turn out to be cystadenomas on final pathology. [9] Therefore, depending on the type of surgery received, this may mean a lifetime of follow-up scans in surveillance of recurrence and cancer.
There is mounting evidence that MRI can be used to pre-operatively differentiates BDH from cystadenomas.[10][11][12][13] On MRI, BDH have well-defined, lobulated margins, thin septations, a thin rim of peripheral enhancement on T2 weighted images, internal fluid content that may contain hemorrhage and/or proteinaceaous material and are without any intracystic vascularized soft tissue elements.[14] By contrast, MRI evaluation of biliary cystadenomas and cystadenocarcinomas show smooth margins, mural nodules, and papillary projections without an enhancing peripheral rim.[15]
Management
The management of BDH has two principal considerations. First, if the patient is asymptomatic, it reasonable to observe the patient and not intervene. Second, if there a confident diagnosis that precludes the possibility of a cystadenoma (which some researchers argue can be achieved using MRI) and patient requires an intervention, the consensus is that the operation of choice is a laparoscopic fenestration. This is usually a brief procedure that involves ‘unroofing’ the cyst with minimal – in any – hepatic resection.[16][17][18] These cysts should not be drained percutaneously as they will only reccur and instrumentation can lead to infection.
Cystadenoma and Cystadenocarcinomas
Less common than BDH, the largest published series describing these lesions found 18 cystadenomas and 4 cystadenocarcinomas at a large institution in Cleveland over the course of 17 years. Roughly 85% are reported in women, aged 41 to 53 years old.[19][20] The key clinical difference with cystadenomas compared to BDH is the chance that they will become malignant.
Radiologically, as above, MRI evaluation of biliary cystadenomas and cystadenocarcinomas show smooth margins, mural nodules, and papillary projections without an enhancing peripheral rim. On pathology, cystadenomas are generally lobulated, multiloculated, and contain clear to mucinous fluid of various colors. The internal lining is generally smooth, composed of a simple columnar or cuboidal glandular epithelium. The key feature that distinguishes the cystadenoma from the BDH is the presence of ovarian-type stroma.[21]>[22] Many, if not most, cystadenomas stain positively for estrogen, progesterone and alpha-inhibin receptors which offer a partially hormonal explanation for the predilection of these lesions for women.[23]
The management of choice for cystadenomas is a complete resection, laparoscopic or open. In the event that a cystadenoma is diagnosed on final pathology and the patient only received a laparoscopic fenestration, the options are either re-operation or long-term follow-up abdominal imaging.
Polycystic Liver Disease
Background
Polycystic liver diseases are genetic disorders that can be either liver-specific or part of a broader disease complex known as autosomal dominant polycystic kidney disease (ADPKD). The defects in pure polycystic liver disease are those of glycoprotein metabolism and thus the lesions are fluid-filled biliary epithelial cysts full of the byproducts of errant oligosaccharide metabolism.[24][25][26] There is recent evidence to suggest that the development of cysts is further exacerbated by a secretin mediated increase in cyclic AMP that generates increased biliary fluid prodcution[27]
The Autosomal Dominant Polycystic Kidney Disease is caused by two mutations in the ADPKD-1 gene on chromosome 16 and ADPKD-2 on chromosome 4. Polycystins, the product of these genes, are proteins expressed on the epithelium of renal tubules, hepatic bile ductules and pancreatic ducts that appear to play crucial roles in the mechanosensation of cilia. [28][29] In other words, these mutations limit the ability of the cells in these tissues to detect and react to structural distortions, leading to cystic degeneration.
Management
The management of these cysts is again driven by the patient’s symptoms – namely pain and discomfort – which are attributed to the scale of a patient’s hepatomegaly. When a patient becomes symptomatic, it is generally held that management ought to be aimed at reducing the liver volume. Still, the vast majority of patients, are asymptomatic and need not pursue any intervention. The standard approach ranges from observation to orthotopic liver transplantation.[30] On the horizon, there is encouraging data that the growth of cysts can be slowed and even reversed to a small degree by secretin-antagonizing somatostatin analogues.[31]
The most conservative – or selective – procedures are cyst aspiration with alcohol sclerosis or hepatic artery embolization. These procedures are typically reserved for patients who are not operative candidates. It has long been known that 100% of aspirated PLD cysts will reoccur.[32] Accordingly, many groups have injected various alcohol (and other) preparations to sclerose the cystic epithelium, with better long-term results.[33] Hepatic artery embolization is well-tolerated but less established and only reduces liver volume by 30% on average.[34]
Operative management is the mainstay of symptomatic cyst therapy. The published rates of symptom recurrence following fenestration are variable depending on the series and are as low as 0% or as high as 100%. The same is true for partial hepatectomy, but the proportion of patients with lasting symptom improvement appears to be reliably higher.[35] Furthermore, volume reduction in cyst fenestration is about 9% on average while partial hepatectomy achieves a 57% reduction.[36] Morbidity, however, tends to be greater for PLD patients undergoing hepatectomy than other patients receiving the same operation.[37]
Finally, liver transplantation is the most radical treatment and is typically reserved for the sickest patients. As observed by one group of clinicians, these patients can develop a syndrome of intractable pain, fatigue and cachexia, that has become known as ‘lethal exhaustion.’[38] Although, their liver function is typically preserved, making their Model for End-Stage Liver Disease (MELD) scores largely insufficient to merit allocation of a transplant. Patients must therefore apply for exceptions on a case-by-case basis.
Outcomes after intervention are dependent on the safety of the procedure and the patient's preoperative condition. Five year survival at a large tertiary referral centre after partial hepatectomy with cyst fenestration, cyst fenestration alone, and liver transplantation was 92%, 90%, and 60%, respectively.[39] Other groups have achieved 80% 5-year survivals in patients receiving liver or combined liver-kidney transplantation. [40][41]
Hydatid Cysts
Hydatid disease – Echinococcosis – is caused by the larval form of Echinococcus granulosus, has a worldwide distribution and is endemic in many developing countries. It is isolated to the liver in 50-70% of patients and the lungs in 20-30%.[42] If symptomatic, patients typically present with pain/discomfort, an abdominal mass, fever or, very rarely, cyst rupture.
Diagnosis
The diagnosis can usually be made by history and physical examination. Basic blood tests are largely unrevealing vis-à-vis liver enzyme profiles and blood counts (eosinophil counts are rarely elevated). Antibody titers (usually IgE) are useful in disseminated disease or tracking recurrence[43]. Indirect immunofluorence (IFA) is the most sensitive (95%), but can cross-react with cystercercosis. Older methods include an intradermal injection of hydatid antigens – the Casoni test – which is positive when a wheal develops. It is up to 90% sensitive in hepatic hydatitosis. The Weinberg test is a complement fixation test that is positive when greater than 1:4. [44]
The diagnosis is usually and reliably made by imaging. Plain film X-rays have an 11% sensitivity so it is advisable to use either ultrasound or CT, which both achieve a sensitivity and specificity of 97-100% and 100%.[45] The management of hydatid cysts is usually based on the imaging findings. The Gharbi classification is depicted below.
Gharbi’s Ultrasound Classification of Hepatic Hydatidosis
- Type 1: Pure fluid collection.
- Type 2: Fluid collection with a split wall.
- Type 3: Fluid collection with a daughter cyst.
- Type 4: Heterogeneous echo pattern.
- Type 5: Calcified wall
Management
The role for an intervention in asymptomatic patients is unclear. The evidence available regarding the natural history of hydatid cysts is sparse, though the little information we have would indicate that more than 75% of asymptomatic patients remain so at 10 years.[46] It is widely agreed that an intervention is indicated for symptomatic hydatid liver cysts and while there are some reports of successful medical treatment with a benzimidazole (albendazole or mebendazole)[47], the gold standard of care is surgery. That said, there has never been a randomized trial of surgical versus medical management.[48]
The interventions at our disposal are percutaneous aspirations (with or without the injection of a sclerosant) – also known as PAIR (puncture, aspiration, injection, and reaspiration) – and operative cyst drainage (laparoscopic or open). Generally, the procedure of choice is guided by the ultrasound-based Gharbi classification of cyst structure[49].
Percutaneous Procedures
Percutaneous procedures were once thought to be dangerous with a risk of anaphylaxis, seeding and spillage. Since 1992, however, there has been a growing body of literature attesting to the safety and efficacy of this procedure.[50][51] In a prospective, randomized trial compared to surgical cystectomy, a percutaneous aspiration (with eight weeks of albendazole) was shown to be as safe and efficacious with a shorter hospital stay.[52] Percutaneous procedures are typically limited to cysts of Type 1 through (and rarely) Type 3.
Operative Management
Surgery can be accomplished by a laparoscopic or open approach. The laparoscopic approach is typically reserved for cysts that are both accessible given standard port placement and have less complicated structures. Any operation on hydatid cysts have two stages. First, the cyst is evacuated and drained. Second, the cyst cavity is addressed. The options for this stage are partial cystectomy with sclerosis and tube drainage, capitonage (using continuous, opposing sutures to obliterate the cystic cavity), omentoplasty (using vascular omentum to patch the cavity) or cystenterostomy (suturing a roux-en-y bypass to the cavity).
There are two main complications specific to procedures for hydatidosis. First, the cyst can reocurr. The rate of recurrence is 3-16% depending on the procedure, with cystectomy and drainage being the operation with the highest risk for reccurrence. Second, biliary injury can occur, either as a leak or fistula. Fistulae can be managed endoscopically or, if unsuccessful, re-operation.[53][54]
(Images courtesy of RadsWiki)
References
- ↑ Caremani M, Vincenti A, Benci A, Sassoli S, Tacconi D. Echographicepidemiology of non-parasitic hepatic cysts. J Clin Ultrasound 1996;21:1 15-1 18.
- ↑ Sanfelippo PM, Beahrs OH,Weiland LH. Cystic disease of the liver. Ann Surg 1974;179:922–925.
- ↑ Sanfelippo PM, Beahrs OH,Weiland LH. Cystic disease of the liver. Ann Surg 1974;179:922–925
- ↑ Brancatelli G, Federle MP, Vilgrain V, Vullierme MP, Marin D, Lagalla R. Fibropolycystic liver disease: CT and MR imaging findings. Radiographics. 2005;25(3):659-670
- ↑ Tapper EB, Adsay NV, Kalb B, Martin D, Kooby D, Sarmiento JM. Symptomatic Bile Duct Hamartomas: Surgical Management in an MRI Driven Practice. J Gastrointest Surg. 2010 May 18. [Epub ahead of print]
- ↑ Regev A et al. Large Cystic Lesions of the Liver in Adults:A 15-Year Experience in a Tertiary Center. J Am Coll Surg 2001;193:36–45
- ↑ Gamblin TC, Holloway SE, Heckman JT, Geller DA. Laparoscopic resection of benign hepatic cysts: a new standard. J Am Coll Surg. Nov 2008;207(5):731-736.
- ↑ Regev A et al. Large Cystic Lesions of the Liver in Adults:A 15-Year Experience in a Tertiary Center. J Am Coll Surg 2001;193:36–45
- ↑ Gamblin TC, Holloway SE, Heckman JT, Geller DA. Laparoscopic resection of benign hepatic cysts: a new standard. J Am Coll Surg. Nov 2008;207(5):731-736.
- ↑ Semelka RC, Hussain SM, Marcos HB, Woosley JT. Biliary hamartomas: solitary and multiple lesions shown on current MR techniques including gadolinium enhancement. J Magn Reson Imaging. 1999;10(2):196-201.
- ↑ Tohme-Noun C, Cazals D, Noun R, Menassa L, Valla D, Vilgrain V. Multiple biliary hamartomas: magnetic resonance features with histopathologic correlation. Eur Radiol. Mar 2008;18(3):493-499.
- ↑ Zheng RQ, Zhang B, Kudo M, Onda H, Inoue T. Imaging findings of biliary hamartomas. World J Gastroenterol 2005; 11:6354-6359.
- ↑ Tapper EB, Adsay NV, Kalb B, Martin D, Kooby D, Sarmiento JM. Symptomatic Bile Duct Hamartomas: Surgical Management in an MRI Driven Practice. Accepted by Journal of Gastrointestinal Surgery (Feb 2010)
- ↑ apper EB, Adsay NV, Kalb B, Martin D, Kooby D, Sarmiento JM. Symptomatic Bile Duct Hamartomas: Surgical Management in an MRI Driven Practice. J Gastrointest Surg. 2010 May 18. [Epub ahead of print]
- ↑ Lewin M, Mourra N, Honigman I, et al. Assessment of MRI and MRCP in diagnosis of biliary cystadenoma and cystadenocarcinoma. Eur Radiol 2006;16(2):407-413
- ↑ Katkhouda A et al. Laparoscopic Management of Benign Solid and Cystic Lesions of the Liver. Annals of Surgery 1999; 229(4):460-466.
- ↑ Gamblin TC, Holloway SE, Heckman JT, Geller DA. Laparoscopic resection of benign hepatic cysts: a new standard. J Am Coll Surg. Nov 2008;207(5):731-736.
- ↑ apper EB, Adsay NV, Kalb B, Martin D, Kooby D, Sarmiento JM. Symptomatic Bile Duct Hamartomas: Surgical Management in an MRI Driven Practice. J Gastrointest Surg. 2010 May 18. [Epub ahead of print]
- ↑ Devaney K, Goodman ZD, Ishak KG. Hepatobiliary cystadenoma and cystadenocarcinoma. A light microscopic and immunohistochemical study of 70 patients. Am J Pathol 1994;18:1078–1091.
- ↑ Vogt et al. Cystadenoma and Cystadenocarcinoma of the Liver:A Single Center Experience. J AmColl Surg 2005;200:727–733
- ↑ Lam MM et al. Ovarian-Type Stroma in Hepatobiliary Cystadenomas and Pancreatic Mucinous Cystic Neoplasms. Am J Clin Pathol 2008;129:211-218
- ↑ Vogt et al. Cystadenoma and Cystadenocarcinoma of the Liver: A Single Center Experience. J AmColl Surg 2005;200:727–733
- ↑ Zamboni G, Scarpa A, Bogina G, et al. Mucinous cystic tumors of the pancreas: clinicopathological features, prognosis, and relationship to other mucinous cystic tumors. Am J SurgPathol. 1999;23:410-422.
- ↑ Torres VE. Treatment of polycystic liver disease: one size does not fit all. Am J Kidney Dis. 2007;49:725–728
- ↑ Davila S, Furu L, Gharavi AG, et al: Mutations in SEC63 cause autosomal dominant polycystic liver disease. Nat Genet 36:575-577, 2004
- ↑ Li A, Davila S, Furu L, et al: Mutations in PRKCSH cause isolated autosomal dominant polycystic liver disease. Am J Hum Genet 72:691-703, 2003
- ↑ Gong AY, Tietz PS, Muff MA, et al. Somatostatin stimulates ductal bile absorption and inhibits ductal bile secretion in mice via SSTR2 on cholangiocytes. Am J Physiol Cell Physiol 2003;284:C1205–C1214
- ↑ Geng L et al. Identification and Localization of Polycystin, the PKD1 Gene Product.J. Clin. Invest.1996. 98:2674–2682
- ↑ Nauli SM et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nature Genetics 2003;33: 129 - 137
- ↑ Arnold HL et al. New Advances in Evaluation and Management of Patients with Polycystic Liver Disease. Am J Gastroenterol 2005;100:2569–2582
- ↑ Van Keimpema L et al. Lanreotide Reduces the Volume of Polycystic Liver: A Randomized, Double-Blind, Placebo-Controlled Trial. Gastroenterology 2009;137:1661–1668
- ↑ Saini S, Mueller P, Ferrucci J, et al. Percutaneous aspiration of hepatic cysts does not provide definitive therapy. Am J Roentgenol 1983;141:559–60.
- ↑ Tikkakoski T, Makela JT, Leinonen S, et al. Treatment of symptomatic congenital hepatic cysts with single session percutaneous drainage and ethanol sclerosis: Technique and outcome. J Vasc Interv Radiol 1996;7:235–9.
- ↑ Takei R, Ubara Y, Hoshino J, et al. Percutaneous transcatheter hepatic artery embolization for liver cysts in autosomal dominant polycystic kidney disease.Am J Kidney Dis. 2007;49:744 –752.
- ↑ Arnold HL et al. New Advances in Evaluation and Management of Patients with Polycystic Liver Disease. Am J Gastroenterol 2005;100:2569–2582
- ↑ Schnelldofger T et al. Polycystic Liver Disease: A Critical Appraisal of Hepatic Resection, Cyst Fenestration,and Liver Transplantation. Ann Surg 2009;250: 112–118)
- ↑ Gali B, Findlay JY, Plevak DJ, et al. Right hepatectomy for living liver donation vs right hepatectomy for disease: intraoperative and immediate postoperative comparison. Arch Surg. 2007;142:467– 471
- ↑ Starzl TE, Reyes J, Tzakis A, et al. Liver transplantation for polycystic liver disease. Arch Surg 1990;125:575–7.
- ↑ Schnelldofger T et al. Polycystic Liver Disease: A Critical Appraisal of Hepatic Resection, Cyst Fenestration,and Liver Transplantation. Ann Surg 2009;250: 112–118)
- ↑ Ueno T, Barri YM, Netto GJ, et al. Liver and kidney transplantation for polycystic liver and kidney-renal function and outcome. Transplantation.2006;82:501–507.
- ↑ Kirchner GI, Rifai K, Cantz T, et al. Outcome and quality of life in patients with polycystic liver disease after liver or combined liver-kidney transplantation.Liver Transplant. 2006;12:1268 –1277.
- ↑ Ammann RW, Eckert J. Cestodes. Echinococcus. Gastroenterol Clin North Am 1996;25:655–689.
- ↑ Force L et al.Evaluation of eight serological tests in the diagnosis of human echinococcosis and follow-up. Clin Infect Dis. 1992 Sep;15(3):473-80
- ↑ Biava MF et al. Laboratory Diagnosis of Cystic Hydatic Disease. World J. Surg 2001;25:10–14
- ↑ Balik AA et al. Surgical Treatment of Hydatid Disease of the Liver: Review of 304 Cases.Arch Surg. 1999;134:166-169
- ↑ Frider B et al. Long-term outcome of asymptomatic liver hydatidosis. J Hepatol 1999;30:228-231
- ↑ Morris DL et al. Albendazole--objective evidence of response in human hydatid disease. JAMA. 1985 Apr 12;253(14):2053-7
- ↑ Nasseri MS et al. Percutaneous needle aspiration, injection, and reaspiration with or without benzimidazole coverage for uncomplicated hepatic hydatid cysts. Cochrane Database Syst Rev. 2006 Apr 19;(2):CD003623.
- ↑ Gharbi HA, Hassine W, Brauner MW, et al. Ultrasound examination of the hydatic liver. Radiology 1981;139:459–463.
- ↑ Ustunoz A et al. Percutaneous Treatment of Hydatid Cysts of the Liver: Long Term Results. AJR 1999;172:91-96
- ↑ Nasseri MS et al. Percutaneous needle aspiration, injection, and reaspiration with or without benzimidazole coverage for uncomplicated hepatic hydatid cysts. Cochrane Database Syst Rev. 2006 Apr 19;(2):CD003623.
- ↑ Kuroo MS. Percutaneous Drainage Compared with Surgery for Hepatic Hydatid Cysts. N Engl J Med 1997;337:881-7.
- ↑ Balik AA et al. Surgical Treatment of Hydatid Disease of the Liver: Review of 304 Cases.Arch Surg. 1999;134:166-169
- ↑ Yagci G et al.Results of Surgical, Laparoscopic, and Percutaneous Treatment for Hydatid Disease of the Liver: 10 Years Experience with 355 Patients. World J Surg (2005) 29: 1670–1679