Gonorrhea medical therapy: Difference between revisions
| Line 68: | Line 68: | ||
**Philadelphia, Pennsylvania (1997) | **Philadelphia, Pennsylvania (1997) | ||
**Oklahoma City, Oklahoma (2012) | **Oklahoma City, Oklahoma (2012) | ||
*from 2006–2012, the prevalence of elevated Ceftriaxone (MICs) was higher in isolates from Men Who have Sex With Men (MSM) than from Men Who have Sex With women (MSW) | |||
===Cefixime=== | ===Cefixime=== | ||
*In 1992, [[Cefixime]] susceptibility testing began and was discontinued in 2007 | *In 1992, [[Cefixime]] susceptibility testing began and was discontinued in 2007 | ||
Revision as of 19:55, 15 September 2016
|
Gonorrhea Microchapters |
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
Gonorrhea medical therapy On the Web |
|
American Roentgen Ray Society Images of Gonorrhea medical therapy |
|
Risk calculators and risk factors for Gonorrhea medical therapy |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Template:Saram
Overview
The mainstay of therapy for gonococcal infections is antimicrobial therapy. Gonorrhea treatment is complicated by the ability of N. gonorrhoeae to develop resistance to antimicrobials, therefore a combination therapy with Azithromycin and a Cephalosporin is used to improve treatment efficacy and potentially slow the emergence and spread of resistance.
Medical Therapy
- The mainstay of therapy for gonococcal infections is antimicrobial therapy.
- Gonorrhea treatment is complicated by the ability of Neisseria gonorrhoeae to develop resistance to antimicrobials, therefore a combination therapy with Azithromycin and a Cephalosporin is used to improve treatment efficacy and potentially slow the emergence and spread of resistance.
- Ceftriaxone and cefixime exist as the last remaining options for empirical first-line treatment of Neisseria gonorrhoeae[1]
| Type of gonococcal infection | Regimen |
|---|---|
| Uncomplicated Recommended regimen |
|
| Uncomplicated Alternative regimen |
|
| Alternative regimens for severe Cephalosporin allergy |
|
| Arthritis and arthritis-dermatitis syndrome |
|
| Gonococcal meningitis and endocarditis |
|
Drug Resistance
- High-level resistance to these expanded-spectrum cephalosporins is now reported, and it seems developing another effective treatment has become unaffordable.
- Although new combination antibiotic treatments are being evaluated. There are no affordable alternative therapeutic options currently available for the treatment of gonococcal disease. and it seems even newly developed antibiotics will be short solution and may be developed resistance as well.
The drug resistance may be developed by following mechanisms:
- Chromosomal mutations
- Acquisition of R factors (plasmids)
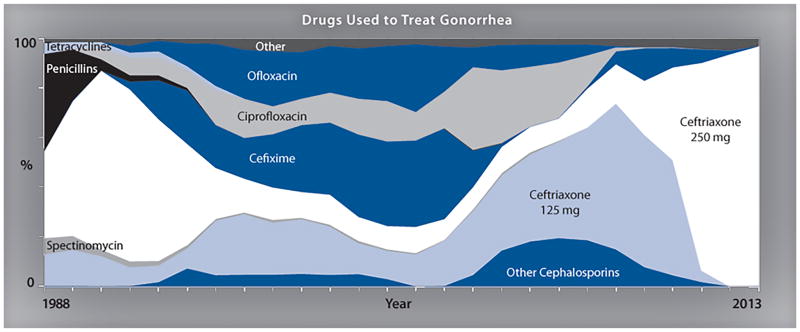
- In 1986, the CDC established the Gonococcal Isolate Surveillance Project (GISP)
- Monitor antimicrobial susceptibility trends in the United States
- Characterize male patients with gonococcal infection
- Phenotypically characterize antimicrobial-resistant isolates of Niesseira gonorrhea

Penicillin
Penicillin has not been a first-line treatment option for gonorrhea for a number of decades. However, it has been tested for surveillance purposes.
- In 2012, Almost 13.1% of Niesseria gonorrhea from the GISP survey were resistant to penicillin mainly with two mechanisms:
- Chromosomal penicillin resistance (82.3%)
- Penicillinase production (17.7%)
Ceftriaxone
- In 1987, susceptibility testing for ceftriaxone began and continues to date.
- From 2008 to 2011, ceftriaxone Minimum inhibitory concentrations MICs increased from 0.1% to 0.4%.
- Five isolates with decreased ceftriaxone susceptibility (MIC 0.5 lg/ml) include:
- San Diego, California (1987)
- Cincinnati, Ohio (1992 and 1993)
- Philadelphia, Pennsylvania (1997)
- Oklahoma City, Oklahoma (2012)
- from 2006–2012, the prevalence of elevated Ceftriaxone (MICs) was higher in isolates from Men Who have Sex With Men (MSM) than from Men Who have Sex With women (MSW)
Cefixime
- In 1992, Cefixime susceptibility testing began and was discontinued in 2007
- In 2009, [Cefixime]] susceptibility testing was restarted due to lack of drug supply in the United States
- From 2006 to 2010, ceftixim Minimum inhibitory concentrations (MICs) increased from 0.1 to 1.4
- from 2006–2012, the prevalence of elevated cefixime (MICs) was higher in isolates from Men Who have Sex With Men (MSM) than from Men Who have Sex With women (MSW)
due to lack of drug supply in the United States, and was restarted in 2009. The percentage of isolates with elevated cefixime MICs increased from 0.1% in 2006 to 1.4% in 2010 and 2011.4 The proportion of iso- lates with elevated cefixime MICs declined to 1.0% in 2012 and further declined to 0.4% in 2013. In 2013, no isolates displayed cefixime MICs of 0.5 lg/ml or higher.4 Similar to ceftriaxone, from 2006–2012, the prevalence of elevated cefixime MICs was higher in isolates from MSM than from MSW. Specifi- cally, isolates with decreased susceptibility from MSM increased from 0% in 2006 to 3.9% in 2010 and then decreased to 2.9% in 2012.9 For the same time period, the proportion with ele- vated MICs from MSWs ranged from 0.1% in 2006 to 0.5% in 2011.
Antimicrobial Regimen
- Neisseria gonorrhoeae treatment[2]
- 1. Gonococcal infections in adolescents and adults
- 1.1 Uncomplicated gonococcal infections of the cervix, urethra, and rectum
- Preferred regimen: Ceftriaxone 250 mg IM in a single dose AND Azithromycin 1 g PO in a single dose
- Alternative regimen: Cefixime 400 mg PO in a single dose AND Azithromycin 1 g PO in a single dose (if ceftriaxone is not available)
- 1.2 Uncomplicated gonococcal infections of the pharynx
- Preferred regimen: Ceftriaxone 250 mg IM in a single dose AND Azithromycin 1 g PO in a single dose
- 1.2.1 Management of sex partners
- Expedited partner therapy: Cefixime 400 mg PO in a single dose AND Azithromycin 1 g PO in a single dose
- Note (1): Recent sex partners (i.e., persons having sexual contact with the infected patient within the 60 days preceding onset of symptoms or gonorrhea diagnosis) should be referred for evaluation, testing, and presumptive dual treatment.
- Note (2): If the patient’s last potential sexual exposure was >60 days before onset of symptoms or diagnosis, the most recent sex partner should be treated.
- Note (3): To avoid reinfection, sex partners should be instructed to abstain from unprotected sexual intercourse for 7 days after they and their sexual partner(s) have completed treatment and after resolution of symptoms, if present.
- 1.2.2 Allergy, intolerance, and adverse reactions
- Preferred regimen (1): Gemifloxacin 320 mg PO in a single dose AND Azithromycin 2 g PO in a single dose
- Preferred regimen (2): Gentamicin 240 mg IM in a single dose AND Azithromycin 2 g PO in a single dose
- Note: Use of Ceftriaxone or Cefixime is contraindicated in persons with a history of an IgE-mediated penicillin allergy (e.g., anaphylaxis, Stevens Johnson syndrome, and toxic epidermal necrolysis).
- 1.2.3 Pregnancy
- Preferred regimen: Ceftriaxone 250 mg IM in a single dose AND Azithromycin 1 g PO in a single dose
- 1.2.4 Suspected cephalosporin treatment failure
- Preferred regimen: Ceftriaxone 250 mg IM in a single dose AND Azithromycin 1 g PO in a single dose
- Alternative regimen (1): Gemifloxacin 320 mg PO single dose AND Azithromycin 2 g PO single dose (when isolates have elevated cephalosporin MICs)
- Alternative regimen (2): Gentamicin 240 mg IM single dose AND Azithromycin 2 g PO single dose (when isolates have elevated cephalosporin MICs)
- Alternative regimen (3): Ceftriaxone 250 mg IM as a single dose AND Azithromycin 2 g PO as a single dose (failure after treatment with Cefixime and Azithromycin)
- Note: Treatment failure should be considered in: (1) patients whose symptoms do not resolve within 3–5 days after appropriate treatment and report no sexual contact during the post-treatment follow-up period; (2) patients with a positive test-of-cure (i.e., positive culture ≥ 72 hours or positive NAAT ≥ 7 days after receiving recommended treatment) when no sexual contact is reported during the post-treatment follow-up period; (3) persons who have a positive culture on test-of-cure (if obtained) if there is evidence of decreased susceptibility to cephalosporins on antimicrobial susceptibility testing, regardless of whether sexual contact is reported during the post-treatment follow-up period.
- 1.3 Gonococcal conjunctivitis
- Preferred regimen: Ceftriaxone 250 mg IM in a single dose AND Azithromycin 1 g PO in a single dose
- Note: Consider one-time lavage of the infected eye with saline solution.
- 1.3.1 Management of sex partners
- Patients should be instructed to refer their sex partners for evaluation and treatment.
- 1.4 Disseminated gonococcal infection
- 1.4.1 Arthritis and arthritis-dermatitis syndrome
- Preferred regimen: Ceftriaxone 1 g IM/IV q24h for 7 days AND Azithromycin 1 g PO in a single dose
- Alternative regimen (1): Cefotaxime 1 g IV q8h for 7 days
- Alternative regimen (2): Ceftizoxime 1 g IV q8h for 7 days AND Azithromycin 1 g PO in a single dose
- 1.4.2 Gonococcal meningitis and endocarditis
- Preferred regimen: Ceftriaxone 1-2 g IV q 12-24 h for 10-14 days AND Azithromycin 1 g PO in a single dose
- 2. Gonococcal infections among neonates
- 2.1 Ophthalmia neonatorum caused by N. gonorrhoeae
- Preferred regimen: Ceftriaxone 25-50 mg/kg IM/IV in a single dose, not to exceed 125 mg
- 2.1.1 Management of mothers and their sex partners
- Mothers of infants with ophthalmia neonatorum caused by N. gonorrhoeae should be evaluated, tested, and presumptively treated for gonorrhea, along with their sex partner(s).
- 2.2 Disseminated gonococcal infection and gonococcal scalp abscesses in neonates
- Preferred regimen (1): Ceftriaxone 25-50 mg/kg/day IM/IV q24h for 7 days
- Preferred regimen (2): Cefotaxime 25 mg/kg IM/IV q12h for 7 days.
- Note (1): The duration of treatment is 10-14 days if meningitis is documented.
- Note (2): Ceftriaxone should be administered cautiously to hyperbilirubinemic infants, especially those born prematurely.
- 2.2.1 Management of mothers and their sex partners
- Mothers of infants who have DGI or scalp abscesses caused by N. gonorrhoeae should be evaluated, tested, and presumptively treated for gonorrhea, along with their sex partner(s).
- 2.3 Neonates born to mothers who have gonococcal infection
- Preferred regimen: Ceftriaxone 25-50 mg/kg IM/IV in a single dose, not to exceed 125 mg
- 2.3.1 Management of mothers and their sex partners
- Mothers who have gonorrhea and their sex partners should be evaluated, tested, and presumptively treated for gonorrhea.
- 3. Gonococcal infections among infants and children
- 3.1 Infants and children who weigh ≤ 45 kg and who have uncomplicated gonococcal vulvovaginitis, cervicitis, urethritis, pharyngitis, or proctitis
- Preferred regimen: Ceftriaxone 25-50 mg/kg IM/IV in a single dose, not to exceed 125 mg
- 3.2 Children who weigh > 45 kg and who have uncomplicated gonococcal vulvovaginitis, cervicitis, urethritis, pharyngitis, or proctitis
- Preferred regimen: Ceftriaxone 250 mg IM in a single dose AND Azithromycin 1g PO in a single dose
- Alternative regimen: Cefixime 400 mg PO single dose AND Azithromycin 1 g PO single dose.(If ceftriaxone is not available)
- 3.3 Children who weigh ≤ 45 kg and who have bacteremia or arthritis
- Preferred regimen: Ceftriaxone 50 mg/kg (maximum dose: 1 g) IM/IV q24h for 7 days
- 3.4 Children who weigh > 45 kg and who have bacteremia or arthritis
- Preferred regimen: Ceftriaxone 1 g IM/IV q24h for 7 days
References
- ↑ Centers for disease control and prevention. Sexually Transmitted Diseases Treatment Guidelines, 2015. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr6403a1.htm Accessed on September 14, 2016
- ↑ Workowski, Kimberly A.; Bolan, Gail A. (2015-06-05). "Sexually transmitted diseases treatment guidelines, 2015". MMWR. Recommendations and reports: Morbidity and mortality weekly report. Recommendations and reports / Centers for Disease Control. 64 (RR-03): 1–137. ISSN 1545-8601. PMID 26042815.