Flecainide
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gerald Chi
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
See full prescribing information for complete Boxed Warning.
Mortality:
Ventricular Pro-Arrhythmic Effects in Patients with Atrial Fibrillation/Flutter:
|
Overview
Flecainide is an antiarrhythmic that is FDA approved for the {{{indicationType}}} of paroxysmal supraventricular tachycardias (PSVT), paroxysmal atrial fibrillation/flutter (PAF) associated with disabling symptoms, sustained ventricular tachycardia (sustained VT). Fecainide is also indicated for the treatment of documented ventricular arrhythmias, such as sustained VT. There is a Black Box Warning for this drug as shown here. Common adverse reactions include palpitations, nausea, dizziness, headache, blurred vision, photopsia, dyspnea, and fatigue.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- For patients with sustained VT, no matter what their cardiac status, flecainide, like other antiarrhythmics, should be initiated in-hospital with rhythm monitoring.
- Flecainide has a long half-life (12 to 27 hours in patients). Steady-state plasma levels, in patients with normal renal and hepatic function, may not be achieved until the patient has received 3 to 5 days of therapy at a given dose. Therefore, increases in dosage should be made no more frequently than once every four days, since during the first 2 to 3 days of therapy the optimal effect of a given dose may not be achieved.
- Intravenous lidocaine has been used occasionally with flecainide while awaiting the therapeutic effect of flecainide. No adverse drug interactions were apparent. However, no formal studies have been performed to demonstrate the usefulness of this regimen.
- An occasional patient not adequately controlled by (or intolerant to) a dose given at 12 hour intervals may be dosed at eight-hour intervals.
- Once adequate control of the arrhythmia has been achieved, it may be possible in some patients to reduce the dose as necessary to minimize side effects or effects on conduction. In such patients, efficacy at the lower dose should be evaluated.
- Flecainide should be used cautiously in patients with a history of CHF or myocardial dysfunction.
- Any use of flecainide in children should be directly supervised by a cardiologist skilled in the treatment of arrhythmias in children. Because of the evolving nature of information in this area, specialized literature should be consulted. Under six months of age, the initial starting dose of flecainide in children is approximately 50 mg/M2 body surface area daily, divided into two or three equally spaced doses. Over six months of age, the initial starting dose may be increased to 100 mg/M2 per day. The maximum recommended dose is 200 mg/M2 per day. This dose should not be exceeded. In some children on higher doses, despite previously low plasma levels, the level has increased rapidly to far above therapeutic values while taking the same dose. Small changes in dose may also lead to disproportionate increases in plasma levels. Plasma trough (less than one hour pre-dose) flecainide levels and electrocardiograms should be obtained at presumed steady state (after at least five doses) either after initiation or change in flecainide dose, whether the dose was increased for lack of effectiveness, or increased growth of the patient. For the first year on therapy, whenever the patient is seen for reasons of clinical follow-up, it is suggested that a 12 lead electrocardiogram and plasma trough flecainide level are obtained. The usual therapeutic level of flecainide in children is 200 to 500 ng/mL. In some cases, levels as high as 800 ng/mL may be required for control.
- In patients with severe renal impairment (creatinine clearance of 35 mL/min/1.73 square meters or less), the initial dosage should be 100 mg once daily (or 50 mg bid); when used in such patients, frequent plasma level monitoring is required to guide dosage adjustments (seePlasma Level Monitoring). In patients with less severe renal disease, the initial dosage should be 100 mg every 12 hours; plasma level monitoring may also be useful in these patients during dosage adjustment. In both groups of patients, dosage increases should be made very cautiously when plasma levels have plateaued (after more than four days), observing the patient closely for signs of adverse cardiac effects or other toxicity. It should be borne in mind that in these patients it may take longer than four days before a new steady-state plasma level is reached following a dosage change.
- Based on theoretical considerations, rather than experimental data, the following suggestion is made: when transferring patients from another antiarrhythmic drug to flecainide allow at least two to four plasma half-lives to elapse for the drug being discontinued before starting flecainide at the usual dosage. In patients where withdrawal of a previous antiarrhythmic agent is likely to produce life-threatening arrhythmias, the physician should consider hospitalizing the patient.
- When flecainide is given in the presence of amiodarone, reduce the usual flecainide dose by 50% and monitor the patient closely for adverse effects. Plasma level monitoring is strongly recommended to guide dosage with such combination therapy.
PSVT and PAF
- Dosing Information
- 50 mg every 12 hours
- Flecainide acetate doses may be increased in increments of 50 mg bid every four days until efficacy is achieved.
- For PAF patients, a substantial increase in efficacy without a substantial increase in discontinuations for adverse experiences may be achieved by increasing the flecainide acetate dose from 50 to 100 mg bid.
- The maximum recommended dose for patients with paroxysmal supraventricular arrhythmias is 300 mg/day.
Sustained VT
- Dosing Information
- 100 mg every 12 hours
- This dose may be increased in increments of 50 mg bid every four days until efficacy is achieved.
- Most patients with sustained VT do not require more than 150 mg every 12 hours (300 mg/day) and the maximum dose recommended is 400 mg/day.
- In patients with sustained VT, use of higher initial doses and more rapid dosage adjustments have resulted in an increased incidence of proarrhythmic events and CHF, particularly during the first few days of dosing. Therefore, a loading dose is not recommended.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Flecainide in adult patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Flecainide in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding FDA-Labeled Use of Flecainide in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Flecainide in pediatric patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Flecainide in pediatric patients.
Contraindications
- Condition1
Warnings
|
See full prescribing information for complete Boxed Warning.
Mortality:
Ventricular Pro-Arrhythmic Effects in Patients with Atrial Fibrillation/Flutter:
|
- Description
Precautions
- Description
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Clinical Trial Experience of Flecainide in the drug label.
Body as a Whole
Cardiovascular
Digestive
Endocrine
Hematologic and Lymphatic
Metabolic and Nutritional
Musculoskeletal
Neurologic
Respiratory
Skin and Hypersensitivy Reactions
Special Senses
Urogenital
Miscellaneous
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Flecainide in the drug label.
Body as a Whole
Cardiovascular
Digestive
Endocrine
Hematologic and Lymphatic
Metabolic and Nutritional
Musculoskeletal
Neurologic
Respiratory
Skin and Hypersensitivy Reactions
Special Senses
Urogenital
Miscellaneous
Drug Interactions
- Drug
- Description
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Flecainide in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Flecainide during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Flecainide with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Flecainide with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Flecainide with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Flecainide with respect to specific gender populations.
Race
There is no FDA guidance on the use of Flecainide with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Flecainide in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Flecainide in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Flecainide in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Flecainide in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
- Plasma Level Monitoring
- The large majority of patients successfully treated with flecainide were found to have trough plasma levels between 0.2 and 1 mcg/mL. The probability of adverse experiences, especially cardiac, may increase with higher trough plasma levels, especially when these exceed 1 mcg/mL. Periodic monitoring of trough plasma levels may be useful in patient management. Plasma level monitoring is required in patients with severe renal failure or severe hepatic disease, since elimination of flecainide from plasma may be markedly slower. Monitoring of plasma levels is strongly recommended in patients on concurrent amiodarone therapy and may also be helpful in patients with CHF and in patients with moderate renal disease.
IV Compatibility
There is limited information regarding IV Compatibility of Flecainide in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- Description
Management
- Description
Chronic Overdose
There is limited information regarding Chronic Overdose of Flecainide in the drug label.
Pharmacology
There is limited information regarding Flecainide Pharmacology in the drug label.
Mechanism of Action
- Flecainide has local anesthetic activity and belongs to the membrane stabilizing (Class 1) group of antiarrhythmic agents; it has electrophysiologic effects characteristic of the IC class of antiarrhythmics.
Electrophysiology
- In man, flecainide produces a dose-related decrease in intracardiac conduction in all parts of the heart with the greatest effect on the His-Purkinje system (H-V conduction). Effects upon atrioventricular (AV) nodal conduction time and intra-atrial conduction times, although present, are less pronounced than those on ventricular conduction velocity. Significant effects on refractory periods were observed only in the ventricle. Sinus node recovery times (corrected) following pacing and spontaneous cycle lengths are somewhat increased. This latter effect may become significant in patients with sinus node dysfunction.
- Flecainide causes a dose-related and plasma-level related decrease in single and multiple PVCs and can suppress recurrence of ventricular tachycardia. In limited studies of patients with a history of ventricular tachycardia, flecainide has been successful 30 to 40% of the time in fully suppressing the inducibility of arrhythmias by programmed electrical stimulation. Based on PVC suppression, it appears that plasma levels of 0.2 to 1 mcg/mL may be needed to obtain the maximal therapeutic effect. It is more difficult to assess the dose needed to suppress serious arrhythmias, but trough plasma levels in patients successfully treated for recurrent ventricular tachycardia were between 0.2 and 1 mcg/mL. Plasma levels above 0.7 to 1 mcg/mL are associated with a higher rate of cardiac adverse experiences such as conduction defects or bradycardia. The relation of plasma levels to proarrhythmic events is not established, but dose reduction in clinical trials of patients with ventricular tachycardia appears to have led to a reduced frequency and severity of such events.
Hemodynamics
- Flecainide does not usually alter heart rate, although bradycardia and tachycardia have been reported occasionally.
- In animals and isolated myocardium, a negative inotropic effect of flecainide has been demonstrated. Decreases in ejection fraction, consistent with a negative inotropic effect, have been observed after single administration of 200 to 250 mg of the drug in man; both increases and decreases in ejection fraction have been encountered during multidose therapy in patients at usual therapeutic doses.
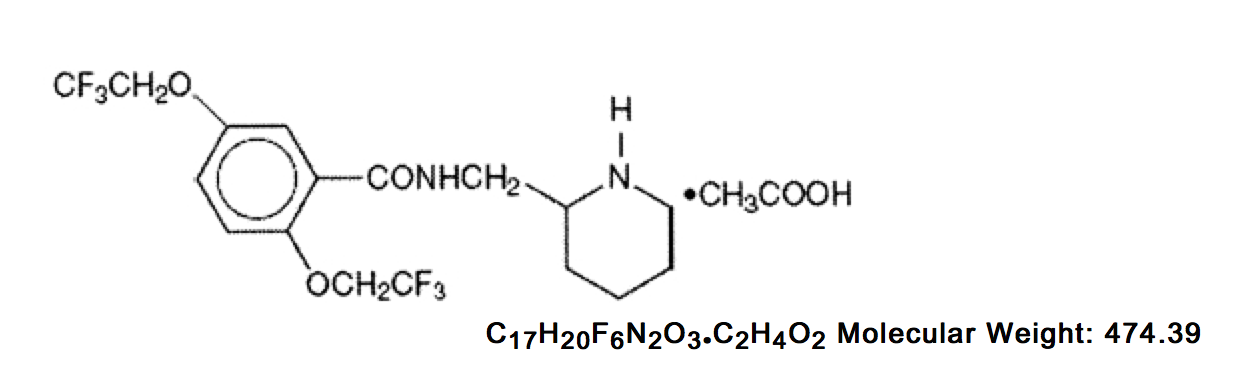
Structure
- Flecainide acetate, USP is an antiarrhythmic drug available in tablets and is benzamide, N-(2-piperidinylmethyl)-2,5-bis(2,2,2-trifluoroethoxy)-monoacetate. The structural formula is as follows:
- Flecainide acetate, USP is a white crystalline substance with a pKa of 9.3. It has an aqueous solubility of 48.4 mg/mL at 37°C.
- Each tablet, for oral administration, contains 50 mg, 100 mg or 150 mg of flecainide acetate, USP. In addition, each tablet contains the following inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, magnesium stearate, microcrystalline celulose, pregelatinized starch and sodium stearyl fumarate.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Flecainide in the drug label.
Pharmacokinetics
- Following oral administration, the absorption of flecainide is nearly complete. Peak plasma levels are attained at about three hours in most individuals (range, 1 to 6 hours). Flecainide does not undergo any consequential presystemic biotransformation (first-pass effect). Food or antacid do not affect absorption. Milk, however, may inhibit absorption in infants. A reduction in flecainide dosage should be considered when milk is removed from the diet of infants.
- The apparent plasma half-life averages about 20 hours and is quite variable (range, 12 to 27 hours) after multiple oral doses in patients with premature ventricular contractions (PVCs). With multiple dosing, plasma levels increase because of its long half-life with steady-state levels approached in 3 to 5 days; once at steady-state, no additional (or unexpected) accumulation of drug in plasma occurs during chronic therapy. Over the usual therapeutic range, data suggest that plasma levels in an individual are approximately proportional to dose, deviating upwards from linearity only slightly (about 10 to 15% per 100 mg on average).
- In healthy subjects, about 30% of a single oral dose (range, 10 to 50%) is excreted in urine as unchanged drug. The two major urinary metabolites are meta-O-dealkylated flecainide (active, but about one-fifth as potent) and the meta-O-dealkylated lactam of flecainide (non-active metabolite). These two metabolites (primarily conjugated) account for most of the remaining portion of the dose. Several minor metabolites (3% of the dose or less) are also found in urine; only 5% of an oral dose is excreted in feces. In patients, free (unconjugated) plasma levels of the two major metabolites are very low (less than 0.05 mcg/mL).
- In vitro metabolic studies have confirmed that cytochrome P450IID6 is involved in the metabolism of flecainide.
- When urinary pH is very alkaline (8 or higher), as may occur in rare conditions (e.g., renal tubular acidosis, strict vegetarian diet), flecainide elimination from plasma is much slower.
- The elimination of flecainide from the body depends on renal function (i.e., 10 to 50% appears in urine as unchanged drug). With increasing renal impairment, the extent of unchanged drug excretion in urine is reduced and the plasma half-life of flecainide is prolonged. Since flecainide is also extensively metabolized, there is no simple relationship between creatinine clearance and the rate of flecanide elimination from plasma.
- In patients with NYHA class III congestive heart failure (CHF), the rate of flecainide elimination from plasma (mean half-life, 19 hours) is moderately slower than for healthy subjects (mean half-life, 14 hours), but similar to the rate for patients with PVCs without CHF. The extent of excre- tion of unchanged drug in urine is also similar.
- Under one year of age, currently available data are limited but suggest that the half-life at birth may be as long as 29 hours, decreasing to 11to 12 hours by three months of age and 6 hours by one year of age. The pharmacokinetics in hydropic infants have not been studied, but case reports suggest prolonged elimination. In children aged 1 year to 12 years the half-life is approximately 8 hours. In adolescents (age 12 to 15) the plasma elimination half-life is approximately 11 to 12 hours. Since milk may inhibit absorption in infants, a reduction in flecainide dosage should be considered when milk is removed from the diet (e.g., gastroenteritis, weaning). Plasma trough flecainide levels should be monitored during major changes in dietary milk intake.
- From age 20 to 80, plasma levels are only slightly higher with advancing age; flecainide elimination from plasma is somewhat slower in elderly subjects than in younger subjects. Patients up to age 80+ have been safely treated with usual dosages.
- The extent of flecainide binding to human plasma proteins is about 40% and is independent of plasma drug level over the range of 0.015 to about 3.4 mcg/mL. Thus, clinically significant drug interactions based on protein binding effects would not be expected.
- Hemodialysis removes only about 1% of an oral dose as unchanged flecainide.
- Small increases in plasma digoxin levels are seen during coadministration of flecainide with digoxin. Small increases in both flecainide and propranolol plasma levels are seen during coadministration of these two drugs.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Flecainide in the drug label.
Clinical Studies
- In two randomized, crossover, placebo-controlled clinical trials of 16 weeks double-blind duration, 79% of patients with paroxysmal supraventricular tachycardia (PSVT) receiving flecainide were attack free, whereas 15% of patients receiving placebo remained attack free. The median time-before-recurrence of PSVT in patients receiving placebo was 11 to 12 days, whereas over 85% of patients receiving flecainide had no recurrence at 60 days.
- In two randomized, crossover, placebo-controlled clinical trials of 16 weeks double-blind duration, 31% of patients with paroxysmal atrial fibrillation/flutter (PAF) receiving flecainide were attack free, whereas 8% receiving placebo remained attack free. The median time-before-recurrence of PAF in patients receiving placebo was about 2 to 3 days, whereas for those receiving flecainide the median time-before-recurrence was 15 days.
How Supplied
- Flecainide Acetate Tablets USP are available as:
- 50 mg: White to off-white, round, flat-faced, beveled-edge, unscored tablet. Debossed with stylized b on one side and 859 on the other side. They are available in bottles of 100 tablets.
- 100 mg: White to off-white, oval, flat-faced, beveled-edge, scored tablet. Debossed with stylized b on one side and 860/100 on the scored side. They are available in bottles of 100 tablets.
- 150 mg: White to off-white, oval, flat-faced, beveled-edge, scored tablet. Debossed with stylized b on one side and 861/150 on the scored side. They are available in bottles of 100 tablets.
- Store at 20º to 25°C (68° to 77°F)
- Dispense in a tight, light-resistant container as defined in the USP, with a child-resistant closure (as required).
- KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
Storage
There is limited information regarding Flecainide Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Flecainide |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Flecainide |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Flecainide in the drug label.
Precautions with Alcohol
- Alcohol-Flecainide interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Tambocor®[1]
Look-Alike Drug Names
- Tambocor® — Pamelor®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "FLECAINIDE ACETATE tablet".
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Flecainide |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Flecainide |Label Name=Flecainide03.png
}}
{{#subobject:
|Label Page=Flecainide |Label Name=Flecainide04.png
}}
{{#subobject:
|Label Page=Flecainide |Label Name=Flecainide05.png
}}
{{#subobject:
|Label Page=Flecainide |Label Name=Flecainide06.png
}}