Etomidate
{{DrugProjectFormSinglePage |authorTag=Chetan Lokhande, M.B.B.S [1] |genericName=Etomidate |aOrAn=a |drugClass=general anesthetic |indication=induction of general anesthesia, maintenance of general anesthesia; adjunct. |adverseReactions=* dermatologic: injection site pain (20%), [[gastrointestinal: nausea, vomiting |blackBoxWarningTitle=TITLE |blackBoxWarningBody=Condition Name: (Content) |fdaLIADAdult=* Induction of general anesthesia: induction, usual dose 0.3 mg/kg IV (range 0.2 to 0.6 mg/kg) over 30 to 60 sec.
- Maintenance of general anesthesia; adjunct: maintenance, 0.01 to 0.02 mg/kg/min IV infusion; dosage must be individualized.
- Procedural sedation: 0.1 to 0.2 mg/kg IV over 30 to 60 seconds, followed by 0.05 mg/kg every 3 to 5 minutes as needed for sedation.
- Rapid sequence intubation, Induction: 0.15 to 0.3 mg/kg IV.
|offLabelAdultGuideSupport=There is limited information about Off-Label Guideline-Supported Use of Etomidate in adult patients. |offLabelAdultNoGuideSupport=There is limited information about Off-Label Non–Guideline-Supported Use of Etomidate in adult patients. |fdaLIADPed=* Not recommended in children under 10 y of age
- Induction of general anesthesia: (children 10 y and older) induction, usual dose 0.3 mg/kg IV (range 0.2 to 0.6 mg/kg) over 30 to 60 sec
- Rapid sequence intubation, Induction: 0.2 to 0.3 mg/kg IV
|offLabelPedGuideSupport=There is limited information about Off-Label Guideline-Supported Use of Etomidate in pediatric patients. |offLabelPedNoGuideSupport=There is limited information about Off-Label Non–Guideline-Supported Use of Etomidate in pediatric patients. |contraindications=Etomidate is contraindicated in patients who have shown hypersensitivity to it. |warnings=
- Intravenous etomidate should be administered only by persons trained in the administration of general anesthetics and in the management of complications encountered during the conduct of general anesthesia.
- Because of the hazards of prolonged suppression of endogenous cortisol and aldosterone production, this formulation is not intended for administration by prolonged infusion.
|clinicalTrials=
- The most frequent adverse reactions associated with use of intravenous etomidate are transient venous pain on injection and transient skeletal muscle movements, including myoclonus
- Transient venous pain was observed immediately following intravenous injection of etomidate in about 20% of the patients, with considerable difference in the reported incidence (1.2% to 42%). This pain is usually described as mild to moderate in severity but it is occasionally judged disturbing. The observation of venous pain is not associated with a more than usual incidence of thrombosis or thrombophlebitis at the injection site. Pain also appears to be less frequently noted when larger, more proximal arm veins are employed and it appears to be more frequently noted when smaller, more distal, hand or wrist veins are employed.
- Transient skeletal muscle movements were noted following use of intravenous etomidate in about 32% of the patients, with considerable difference in the reported incidence (22.7% to 63%). Most of these observations were judged mild to moderate in severity but some were judged disturbing. The incidence of disturbing movements was less when 0.1 mg of fentanyl was given immediately before induction. These movements have been classified as myoclonic in the majority of cases (74%), but averting movements (7%), tonic movements (10%), and eye movements (9%) have also been reported. No exact classification is available, but these movements may also be placed into three groups by location:
- Most movements are bilateral. The arms, legs, shoulders, neck, chest wall, trunk and all four extremities have been described in some cases, with one or more of these muscle groups predominating in each individual case. Results of electroencephalographic studies suggest that these muscle movements are a manifestation of disinhibition of cortical activity; cortical electroencephalograms, taken during periods when these muscle movements were observed, have failed to reveal seizure activity.
- Other movements are described as either unilateral or having a predominance of activity of one side over the other. These movements sometimes resemble a localized response to some stimuli, such as venous pain on injection, in the lightly anesthetized patient (averting movements). Any muscle group or groups may be involved, but a predominance of movement of the arm in which the intravenous infusion is started is frequently noted.
- Still other movements probably represent a mixture of the first two types.
- Skeletal muscle movements appear to be more frequent in patients who also manifest venous pain on injection.
Other Adverse Observations
=Respiratory System
- Hyperventilation, hypoventilation, apnea of short duration (5 to 90 seconds with spontaneous recovery); laryngospasm, hiccup and snoring suggestive of partial upper airway obstruction have been observed in some patients. These conditions were managed by conventional countermeasures.
Circulatory System
- Hypertension, hypotension, tachycardia, bradycardia and other arrhythmias have occasionally been observed during induction and maintenance of anesthesia. One case of severe hypotension and tachycardia, judged to be anaphylactoid in character, has been reported.
- Geriatric patients, particularly those with hypertension, may be at increased risk for the development of cardiac depression following etomidate administration (see Clinical pharmacology).
Gastrointestinal System
- Postoperative nausea and/or vomiting following induction of anesthesia with etomidate is probably no more frequent than the general incidence. When etomidate was used for both induction and maintenance of anesthesia in short procedures such as dilation and curettage, or when insufficient analgesia was provided, the incidence of postoperative nausea and/or vomiting was higher than that noted in control patients who received thiopental.
|FDAPregCat=C |useInPregnancyFDA=* Etomidate has been shown to have an embryocidal effect in rats when given in doses 1 and 4 times the human dose. There are no adequate and well-controlled studies in pregnant women. Etomidate should be used during pregnancy only if the potential benefit justifies the potential risks to the fetus. Etomidate has not been shown to be teratogenic in animals. Reproduction studies with etomidate have been shown to:
- Decrease pup survival at 0.3 and 5 mg/kg in rats (approximately 1X and 16X human dosage) and at 1.5 and 4.5 mg/kg in rabbits (approximately 5X and 15X human dosage). No clear dose-related pattern was observed.
- Increase slightly the number of stillborn fetuses in rats at 0.3 and 1.25 mg/kg (approximately 1X and 4X human dosage).
- Cause maternal toxicity with deaths of 6/20 rats at 5 mg/kg (approximately 16X human dosage) and 6/20 rabbits at 4.5 mg/kg (approximately 15X human dosage).
|useInLaborDelivery=* There are insufficient data to support use of intravenous etomidate in obstetrics, including Caesarean section deliveries. Therefore, such use is not recommended. |useInNursing=* It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when etomidate is administered to a nursing mother. |useInPed=* There are inadequate data to make dosage recommendations for induction of anesthesia in patients below the age of ten (10) years; therefore, such use is not recommended (see also Dosage And Administration). |useInGeri=* Clinical data indicates that etomidate may induce cardiac depression in elderly patients, particularly those with hypertension (see Clinical Pharmacology And Other Adverse ObservationS, Circulatory System).
- Elderly patients may require lower doses of etomidate than younger patients. Age-related differences in phamacokinetic parameters have been observed in clinical studies (see Clinical Pharmacology And Dosage And Administration).
- This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection and it may be useful to monitor renal function.
|overdose=* Overdosage may occur from too rapid or repeated injections. Too rapid injection may be followed by a fall in blood pressure. No adverse cardiovascular or respiratory effects attributable to etomidate overdose have been reported.
- In the event of suspected or apparent overdosage, the drug should be discontinued, a patent airway established (intubate, if necessary) or maintained and oxygen administered with assisted ventilation, if necessary.
The LD50 of etomidate administered intravenously to rats is 20.4 mg/kg.
|drugBox=
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Monograph |
| Pregnancy category |
|
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 75% |
| Metabolism | Ester hydrolysis |
| Elimination half-life | 75 minutes |
| Excretion | Renal (85%) and biliary (15%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C14H16N2O2 |
| Molar mass | 244.289 g/mol |
| 3D model (JSmol) | |
| Boiling point | 392 °C (737.6 °F) |
| |
| |
| (verify) | |
|structure=* Amidate (etomidate injection) is a sterile, nonpyrogenic solution. Each milliliter contains etomidate, 2 mg, propylene glycol 35% v/v. The pH is 6.0 (4.0 to 7.0).
- It is intended for the induction of general anesthesia by intravenous injection.
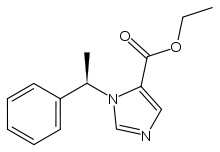
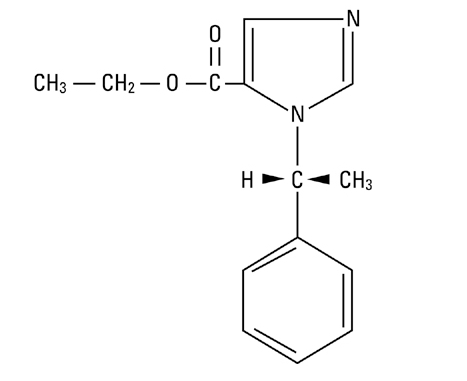
- The drug etomidate is chemically identified as (R)-(+)-ethyl-1-(1-phenylethyl)-1H-imidazole -5-carboxylate and has the following structural formula:
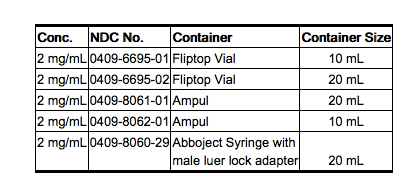
|howSupplied=* Amidate™ (etomidate injection) is supplied in single-dose containers as follows:
|storage=* Store at 20 to 25°C (68 to 77°F). [See USP Controlled Room Temperature.]
- Revised: November, 2009
- Printed in USA EN-2288
- Hospira, Inc., Lake Forest, IL 60045 USA
|alcohol=Alcohol-Etomidate interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. }} {{#subobject:
|Label Page=Etomidate |Label Name=Etomidate label.png
}}