EpiPen: Difference between revisions
m (Robot: Automated text replacement (-{{WikiDoc Cardiology Network Infobox}} +, -<references /> +{{reflist|2}}, -{{reflist}} +{{reflist|2}})) |
No edit summary |
||
| Line 1: | Line 1: | ||
{{ | {{DrugProjectFormSinglePage | ||
|authorTag= | |||
{{VP}} | |||
<!--Overview--> | |||
|genericName= | |||
Epinephrine | |||
|aOrAn= | |||
a | |||
|drugClass= | |||
non-selective alpha and [[beta-adrenergic]] receptor agonist | |||
|indication= | |||
[[allergic]] reactions (Type I) including [[anaphylaxis]] | |||
= | |hasBlackBoxWarning= | ||
|adverseReactions= | |||
{{ | [[anxiety]], apprehensiveness, restlessness, [[tremor]], [[weakness]], [[dizziness]], [[sweating]], [[palpitations]], [[pallor]], [[nausea]] and [[vomiting]], [[headache]], and/or respiratory difficulties. | ||
{{ | |||
{{ | <!--Black Box Warning--> | ||
|blackBoxWarningTitle= | |||
Title | |||
|blackBoxWarningBody= | |||
<i><span style="color:#FF0000;">ConditionName: </span></i> | |||
* Content | |||
<!--Adult Indications and Dosage--> | |||
<!--FDA-Labeled Indications and Dosage (Adult)--> | |||
|fdaLIADAdult= | |||
=====Emergency Treatment Of Allergic Reactions (Type I)===== | |||
*EpiPen and EpiPen Jr are indicated in the emergency treatment of [[allergic reactions]] (Type I) including [[anaphylaxis]] to stinging insects (e.g., order Hymenoptera, which include bees, wasps, hornets, yellow jackets and fire ants) and biting insects (e.g., triatoma, mosquitoes), allergen [[immunotherapy]], foods, drugs, diagnostic testing substances (e.g., [[radiocontrast]] media) and other allergens, as well as idiopathic [[anaphylaxis]] or exercise-induced [[anaphylaxis]]. | |||
*EpiPen and EpiPen Jr are intended for immediate administration in patients who are determined to be at increased risk for [[anaphylaxis]], including individuals with a history of [[anaphylactic reactions]]. | |||
*Anaphylactic reactions may occur within minutes after exposure and consist of [[flushing]], apprehension, [[syncope]], [[tachycardia]], thready or unobtainable [[pulse]] associated with a fall in [[blood pressure]], [[convulsions]], [[vomiting]], [[diarrhea]] and [[abdominal cramps]], [[involuntary voiding]], [[wheezing]], [[dyspnea]] due to [[laryngeal spasm]], [[pruritus]], [[rashes]], [[urticaria]] or [[angioedema]]. | |||
*EpiPen and EpiPen Jr are intended for immediate administration as emergency supportive therapy only and are not a substitute for immediate medical care. | |||
*Dosing Information | |||
:*Selection of the appropriate dosage strength (EpiPen 0.3 mg or EpiPen Jr 0.15 mg) is determined according to patient body weight. | |||
:**Patients greater than or equal to 30 kg (approximately 66 pounds or more): EpiPen 0.3 mg | |||
:**Patients 15 to 30 kg (33 pounds to 66 pounds): EpiPen Jr 0.15 mg | |||
:*Inject EpiPen or EpiPen Jr intramuscularly or subcutaneously into the anterolateral aspect of the thigh, through clothing if necessary. | |||
Each EpiPen or EpiPen Jr contains a single dose of epinephrine for single-use injection. Since the doses of epinephrine delivered from EpiPen or :*EpiPen Jr are fixed, consider using other forms of injectable epinephrine if doses lower than 0.15 mg are deemed necessary. | |||
<!--Off-Label Use and Dosage (Adult)--> | |||
<!--Guideline-Supported Use (Adult)--> | |||
|offLabelAdultGuideSupport= | |||
=====Condition1===== | |||
* Developed by: | |||
* Class of Recommendation: | |||
* Strength of Evidence: | |||
* Dosing Information | |||
:* Dosage | |||
=====Condition2===== | |||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
<!--Non–Guideline-Supported Use (Adult)--> | |||
|offLabelAdultNoGuideSupport= | |||
=====Condition1===== | |||
* Dosing Information | |||
:* Dosage | |||
=====Condition2===== | |||
There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
<!--Pediatric Indications and Dosage--> | |||
<!--FDA-Labeled Indications and Dosage (Pediatric)--> | |||
|fdaLIADPed= | |||
=====Condition1===== | |||
* Dosing Information | |||
:* Dosage | |||
=====Condition2===== | |||
There is limited information regarding <i>FDA-Labeled Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Off-Label Use and Dosage (Pediatric)--> | |||
<!--Guideline-Supported Use (Pediatric)--> | |||
|offLabelPedGuideSupport= | |||
=====Condition1===== | |||
* Developed by: | |||
* Class of Recommendation: | |||
* Strength of Evidence: | |||
* Dosing Information | |||
:* Dosage | |||
=====Condition2===== | |||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Non–Guideline-Supported Use (Pediatric)--> | |||
|offLabelPedNoGuideSupport= | |||
=====Condition1===== | |||
* Dosing Information | |||
:* Dosage | |||
=====Condition2===== | |||
There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Contraindications--> | |||
|contraindications= | |||
*None | |||
<!--Warnings--> | |||
|warnings= | |||
====Precautions==== | |||
*Emergency Treatment | |||
:*EpiPen and EpiPen Jr are intended for immediate administration as emergency supportive therapy and are not intended as a substitute for immediate medical care. In conjunction with the administration of epinephrine, the patient should seek immediate medical or hospital care. More than two sequential doses of epinephrine should only be administered under direct medical supervision. | |||
*Incorrect Locations of Injection | |||
:*EpiPen and EpiPen Jr should only be injected into the anterolateral aspect of the thigh [see Dosage and Administration (2) and Patient Counseling Information (17)]. | |||
:**Do not inject intravenously. Large doses or accidental intravenous injection of epinephrine may result in cerebral hemorrhage due to sharp rise in blood pressure. Rapidly acting vasodilators can counteract the marked pressor effects of epinephrine if there is such inadvertent administration. | |||
:**Do not inject into buttock. Injection into the buttock may not provide effective treatment of anaphylaxis. Advise the patient to go immediately to the nearest emergency room for further treatment of anaphylaxis. Additionally, injection into the buttock has been associated with gas gangrene. Cleansing with alcohol does not kill bacterial spores, and therefore, does not lower this risk. | |||
:**Do not inject into digits, hands or feet. Since epinephrine is a strong vasoconstrictor, accidental injection into the digits, hands or feet may result in loss of blood flow to the affected area. Advise the patient to go immediately to the nearest emergency room and to inform the healthcare provider in the emergency room of the location of the accidental injection. Treatment of such inadvertent administration should consist of vasodilation, in addition to further appropriate treatment of anaphylaxis [ see Adverse Reactions (6)]. | |||
*Allergic Reactions Associated With Sulfite | |||
:*The presence of a sulfite in this product should not deter administration of the drug for treatment of serious allergic or other emergency situations even if the patient is sulfite-sensitive. | |||
:*Epinephrine is the preferred treatment for serious allergic reactions or other emergency situations even though this product contains sodium metabisulfite, a sulfite that may, in other products, cause allergic-type reactions including anaphylactic symptoms or life-threatening or less severe asthmatic episodes in certain susceptible persons. | |||
:*The alternatives to using epinephrine in a life-threatening situation may not be satisfactory. | |||
*Disease Interactions | |||
:*Some patients may be at greater risk for developing adverse reactions after epinephrine administration. Despite these concerns, it should be recognized that the presence of these conditions is not a contraindication to epinephrine administration in an acute, life-threatening situation. Therefore, patients with these conditions, and/or any other person who might be in a position to administer EpiPen or EpiPen Jr to a patient experiencing anaphylaxis should be carefully instructed in regard to the circumstances under which epinephrine should be used. | |||
*Patients with Heart Disease | |||
:*Epinephrine should be administered with caution to patients who have heart disease, including patients with cardiac arrhythmias, coronary artery or organic heart disease, or hypertension. In such patients, or in patients who are on drugs that may sensitize the heart to arrhythmias, epinephrine may precipitate or aggravate angina pectoris as well as produce ventricular arrhythmias. | |||
*Other Patients and Diseases | |||
:*Epinephrine should be administered with caution to patients with hyperthyroidism, diabetes, elderly individuals, and pregnant women. Patients with Parkinson’s disease may notice a temporary worsening of symptoms. | |||
<!--Adverse Reactions--> | |||
<!--Clinical Trials Experience--> | |||
|clinicalTrials= | |||
*Due to the lack of randomized, controlled clinical trials of epinephrine for the treatment of anaphylaxis, the true incidence of adverse reactions associated with the systemic use of epinephrine is difficult to determine. Adverse reactions reported in observational trials, case reports, and studies are listed below. | |||
*Common adverse reactions to systemically administered epinephrine include anxiety; apprehensiveness; restlessness; tremor; weakness; dizziness; sweating; palpitations; pallor; nausea and vomiting; headache; and/or respiratory difficulties. These symptoms occur in some persons receiving therapeutic doses of epinephrine, but are more likely to occur in patients with hypertension or hyperthyroidism [see Warnings and Precautions (5.4)]. | |||
*Arrhythmias, including fatal ventricular fibrillation, have been reported, particularly in patients with underlying cardiac disease or those receiving certain drugs [see Warnings and Precautions (5.4) and Drug Interactions (7)]. | |||
*Rapid rises in blood pressure have produced cerebral hemorrhage, particularly in elderly patients with cardiovascular disease [see Warnings and Precautions (5.4)]. | |||
*Angina may occur in patients with coronary artery disease [see Warnings and Precautions (5.4)]. | |||
*Accidental injection into the digits, hands or feet may result in loss of blood flow to the affected area [see Warnings and Precautions (5.2)]. | |||
*Adverse events experienced as a result of accidental injections may include increased heart rate, local reactions including injection site pallor, coldness and hypoesthesia or injury at the injection site resulting in bruising, bleeding, discoloration, erythema or skeletal injury. | |||
*Injection into the buttock has resulted in cases of gas gangrene. | |||
<!--Postmarketing Experience--> | |||
|postmarketing= | |||
There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | |||
<!--Drug Interactions--> | |||
|drugInteractions= | |||
*Patients who receive epinephrine while concomitantly taking cardiac glycosides, diuretics, or anti-arrhythmics should be observed carefully for the development of cardiac arrhythmias [see Warnings and Precautions (5.4)]. | |||
*The effects of epinephrine may be potentiated by tricyclic antidepressants, monoamine oxidase inhibitors, levothyroxine sodium, and certain antihistamines, notably chlorpheniramine, tripelennamine, and diphenhydramine. | |||
*The cardiostimulating and bronchodilating effects of epinephrine are antagonized by beta- adrenergic blocking drugs, such as propranolol. | |||
*The vasoconstricting and hypertensive effects of epinephrine are antagonized by alpha- adrenergic blocking drugs, such as phentolamine. | |||
*Ergot alkaloids may also reverse the pressor effects of epinephrine. | |||
<!--Use in Specific Populations--> | |||
|useInPregnancyFDA= | |||
* '''Pregnancy Category C''' | |||
*There are no adequate and well controlled studies of the acute effect of epinephrine in pregnant women. | |||
*Epinephrine was teratogenic in rabbits, mice and hamsters. Epinephrine should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus (fetal anoxia, spontaneous abortion, or both). | |||
*Epinephrine has been shown to have teratogenic effects when administered subcutaneously in rabbits at approximately 30 times the maximum recommended daily subcutaneous or intramuscular dose (on a mg/m2 basis at a maternal dose of 1.2 mg/kg/day for two to three days), in mice at approximately 7 times the maximum daily subcutaneous or intramuscular dose (on a mg/m2 basis at a maternal subcutaneous dose of 1 mg/kg/day for 10 days), and in hamsters at approximately 5 times the maximum recommended daily subcutaneous or intramuscular dose (on a mg/m2 basis at a maternal subcutaneous dose of 0.5 mg/kg/day for 4 days). | |||
*These effects were not seen in mice at approximately 3 times the maximum recommended daily subcutaneous or intramuscular dose (on a mg/m2 basis at a subcutaneous maternal dose of 0.5 mg/kg/day for 10 days). | |||
|useInPregnancyAUS= | |||
* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | |||
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | |||
|useInLaborDelivery= | |||
There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | |||
|useInNursing= | |||
*It is not known whether epinephrine is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when EpiPen is administered to a nursing woman. | |||
|useInPed= | |||
*EpiPen or EpiPen Jr may be administered to pediatric patients at a dosage appropriate to body weight. Clinical experience with the use of epinephrine suggests that the adverse reactions seen in children are similar in nature and extent to those both expected and reported in adults. Since the doses of epinephrine delivered from EpiPen and EpiPen Jr are fixed, consider using other forms of injectable epinephrine if doses lower than 0.15 mg are deemed necessary. | |||
|useInGeri= | |||
*Clinical studies for the treatment of anaphylaxis have not been performed in subjects aged 65 and over to determine whether they respond differently from younger subjects. However, other reported clinical experience with use of epinephrine for the treatment of anaphylaxis has identified that geriatric patients may be particularly sensitive to the effects of epinephrine. Therefore, EpiPen should be administered with caution in elderly individuals, who may be at greater risk for developing adverse reactions after epinephrine administration. | |||
|useInGender= | |||
There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | |||
|useInRace= | |||
There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | |||
|useInRenalImpair= | |||
There is no FDA guidance on the use of {{PAGENAME}} in patients with renal impairment. | |||
|useInHepaticImpair= | |||
There is no FDA guidance on the use of {{PAGENAME}} in patients with hepatic impairment. | |||
|useInReproPotential= | |||
There is no FDA guidance on the use of {{PAGENAME}} in women of reproductive potentials and males. | |||
|useInImmunocomp= | |||
There is no FDA guidance one the use of {{PAGENAME}} in patients who are immunocompromised. | |||
<!--Administration and Monitoring--> | |||
|administration= | |||
* Intramuscular | |||
|monitoring= | |||
There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | |||
<!--IV Compatibility--> | |||
|IVCompat= | |||
There is limited information regarding <i>IV Compatibility</i> of {{PAGENAME}} in the drug label. | |||
<!--Overdosage--> | |||
|overdose= | |||
===Acute Overdose=== | |||
====Signs and Symptoms==== | |||
*Overdosage of epinephrine may produce extremely elevated arterial pressure, which may result in cerebrovascular hemorrhage, particularly in elderly patients. Overdosage may also result in pulmonary edema because of peripheral vascular constriction together with cardiac stimulation. Treatment consists of rapidly acting vasodilators or alpha-adrenergic blocking drugs and/or respiratory support. | |||
*Epinephrine overdosage can also cause transient bradycardia followed by tachycardia, and these may be accompanied by potentially fatal cardiac arrhythmias. Premature ventricular contractions may appear within one minute after injection and may be followed by multifocal ventricular tachycardia (prefibrillation rhythm). Subsidence of the ventricular effects may be followed by atrial tachycardia and occasionally by atrioventricular block. Treatment of arrhythmias consists of administration of a beta-adrenergic blocking drug such as propranolol. | |||
*Overdosage sometimes results in extreme pallor and coldness of the skin, metabolic acidosis, and kidney failure. | |||
====Management==== | |||
*Suitable corrective measures must be taken in such situations. | |||
===Chronic Overdose=== | |||
There is limited information regarding <i>Chronic Overdose</i> of {{PAGENAME}} in the drug label. | |||
<!--Pharmacology--> | |||
<!--Drug box 2--> | |||
|drugBox= | |||
{{drugbox2 | |||
| Watchedfields = changed | |||
| verifiedrevid = 464189734 | |||
| IUPAC_name = (''R'')-4-(1-Hydroxy-2-(methylamino)ethyl)benzene-1,2-diol | |||
| image = EpiPen.png | |||
| width = 180px | |||
| | |||
| pregnancy_US = C | |||
| legal_AU = S4 | |||
| legal_UK = POM | |||
| legal_US = OTC | |||
| routes_of_administration = [[intravenous|IV]], [[intramuscular|IM]], [[endotracheal tube|endotracheal]], [[Intracardiac injection|IC]], [[Nasal administration|Nasal]] [[Eye drop|Ophthalmic]] | |||
<!--Pharmacokinetic data--> | |||
| bioavailability = | |||
| metabolism = [[synapse|adrenergic synapse]] ([[Monoamine oxidase|MAO]] and [[Catechol-O-methyl transferase|COMT]]) | |||
| elimination_half-life = 2 minutes | |||
| excretion = Urine | |||
<!--Identifiers--> | |||
| CASNo_Ref = {{cascite|correct|CAS}} | |||
| CAS_number_Ref = {{cascite|correct|??}} | |||
| CAS_number = 51-43-4 | |||
| ATC_prefix = A01 | |||
| ATC_suffix = AD01 | |||
| ATC_supplemental = {{ATC|B02|BC09}} {{ATC|C01|CA24}} {{ATC|R01|AA14}} {{ATC|R03|AA01}} {{ATC|S01|EA01}} | |||
| ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| ChEBI = 28918 | |||
| PubChem = 5816 | |||
| IUPHAR_ligand = 479 | |||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| DrugBank = DB00668 | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 5611 | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = YKH834O4BH | |||
| KEGG_Ref = {{keggcite|correct|kegg}} | |||
| KEGG = D00095 | |||
| ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| ChEMBL = 679 | |||
| PDB_ligand = ALE | |||
<!--Chemical data--> | |||
| C=9 | H=13 | N=1 | O=3 | |||
| molecular_weight = 183.204 g/mol | |||
| smiles = Oc1ccc(cc1O)[C@@H](O)CNC | |||
| InChI = 1/C9H13NO3/c1-10-5-9(13)6-2-3-7(11)8(12)4-6/h2-4,9-13H,5H2,1H3/t9-/m0/s1 | |||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChI = 1S/C9H13NO3/c1-10-5-9(13)6-2-3-7(11)8(12)4-6/h2-4,9-13H,5H2,1H3/t9-/m0/s1 | |||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChIKey = UCTWMZQNUQWSLP-VIFPVBQESA-N | |||
}} | |||
<!--Mechanism of Action--> | |||
|mechAction= | |||
* | |||
<!--Structure--> | |||
|structure= | |||
* | |||
: [[File:{{PAGENAME}}01.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
<!--Pharmacodynamics--> | |||
|PD= | |||
There is limited information regarding <i>Pharmacodynamics</i> of {{PAGENAME}} in the drug label. | |||
<!--Pharmacokinetics--> | |||
|PK= | |||
There is limited information regarding <i>Pharmacokinetics</i> of {{PAGENAME}} in the drug label. | |||
<!--Nonclinical Toxicology--> | |||
|nonClinToxic= | |||
There is limited information regarding <i>Nonclinical Toxicology</i> of {{PAGENAME}} in the drug label. | |||
<!--Clinical Studies--> | |||
|clinicalStudies= | |||
There is limited information regarding <i>Clinical Studies</i> of {{PAGENAME}} in the drug label. | |||
<!--How Supplied--> | |||
|howSupplied= | |||
* | |||
<!--Patient Counseling Information--> | |||
|fdaPatientInfo= | |||
There is limited information regarding <i>Patient Counseling Information</i> of {{PAGENAME}} in the drug label. | |||
<!--Precautions with Alcohol--> | |||
|alcohol= | |||
* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |||
<!--Brand Names--> | |||
|brandNames= | |||
* ®<ref>{{Cite web | title = | url = }}</ref> | |||
<!--Look-Alike Drug Names--> | |||
|lookAlike= | |||
* A® — B®<ref name="www.ismp.org">{{Cite web | last = | first = | title = http://www.ismp.org | url = http://www.ismp.org | publisher = | date = }}</ref> | |||
<!--Drug Shortage Status--> | |||
|drugShortage= | |||
}} | |||
<!--Pill Image--> | |||
{{PillImage | |||
|fileName=No image.jpg|This image is provided by the National Library of Medicine. | |||
|drugName= | |||
|NDC= | |||
|drugAuthor= | |||
|ingredients= | |||
|pillImprint= | |||
|dosageValue= | |||
|dosageUnit= | |||
|pillColor= | |||
|pillShape= | |||
|pillSize= | |||
|pillScore= | |||
}} | |||
<!--Label Display Image--> | |||
{{LabelImage | |||
|fileName={{PAGENAME}}11.png|This image is provided by the National Library of Medicine. | |||
}} | |||
{{LabelImage | |||
|fileName={{PAGENAME}}11.png|This image is provided by the National Library of Medicine. | |||
}} | |||
<!--Category--> | |||
[[Category:Drug]] | |||
Revision as of 15:30, 10 October 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Vignesh Ponnusamy, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
EpiPen is a non-selective alpha and beta-adrenergic receptor agonist that is FDA approved for the {{{indicationType}}} of allergic reactions (Type I) including anaphylaxis. Common adverse reactions include anxiety, apprehensiveness, restlessness, tremor, weakness, dizziness, sweating, palpitations, pallor, nausea and vomiting, headache, and/or respiratory difficulties..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Emergency Treatment Of Allergic Reactions (Type I)
- EpiPen and EpiPen Jr are indicated in the emergency treatment of allergic reactions (Type I) including anaphylaxis to stinging insects (e.g., order Hymenoptera, which include bees, wasps, hornets, yellow jackets and fire ants) and biting insects (e.g., triatoma, mosquitoes), allergen immunotherapy, foods, drugs, diagnostic testing substances (e.g., radiocontrast media) and other allergens, as well as idiopathic anaphylaxis or exercise-induced anaphylaxis.
- EpiPen and EpiPen Jr are intended for immediate administration in patients who are determined to be at increased risk for anaphylaxis, including individuals with a history of anaphylactic reactions.
- Anaphylactic reactions may occur within minutes after exposure and consist of flushing, apprehension, syncope, tachycardia, thready or unobtainable pulse associated with a fall in blood pressure, convulsions, vomiting, diarrhea and abdominal cramps, involuntary voiding, wheezing, dyspnea due to laryngeal spasm, pruritus, rashes, urticaria or angioedema.
- EpiPen and EpiPen Jr are intended for immediate administration as emergency supportive therapy only and are not a substitute for immediate medical care.
- Dosing Information
- Selection of the appropriate dosage strength (EpiPen 0.3 mg or EpiPen Jr 0.15 mg) is determined according to patient body weight.
- Patients greater than or equal to 30 kg (approximately 66 pounds or more): EpiPen 0.3 mg
- Patients 15 to 30 kg (33 pounds to 66 pounds): EpiPen Jr 0.15 mg
- Inject EpiPen or EpiPen Jr intramuscularly or subcutaneously into the anterolateral aspect of the thigh, through clothing if necessary.
- Selection of the appropriate dosage strength (EpiPen 0.3 mg or EpiPen Jr 0.15 mg) is determined according to patient body weight.
Each EpiPen or EpiPen Jr contains a single dose of epinephrine for single-use injection. Since the doses of epinephrine delivered from EpiPen or :*EpiPen Jr are fixed, consider using other forms of injectable epinephrine if doses lower than 0.15 mg are deemed necessary.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of EpiPen in adult patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of EpiPen in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding FDA-Labeled Use of EpiPen in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of EpiPen in pediatric patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of EpiPen in pediatric patients.
Contraindications
- None
Warnings
Precautions
- Emergency Treatment
- EpiPen and EpiPen Jr are intended for immediate administration as emergency supportive therapy and are not intended as a substitute for immediate medical care. In conjunction with the administration of epinephrine, the patient should seek immediate medical or hospital care. More than two sequential doses of epinephrine should only be administered under direct medical supervision.
- Incorrect Locations of Injection
- EpiPen and EpiPen Jr should only be injected into the anterolateral aspect of the thigh [see Dosage and Administration (2) and Patient Counseling Information (17)].
- Do not inject intravenously. Large doses or accidental intravenous injection of epinephrine may result in cerebral hemorrhage due to sharp rise in blood pressure. Rapidly acting vasodilators can counteract the marked pressor effects of epinephrine if there is such inadvertent administration.
- Do not inject into buttock. Injection into the buttock may not provide effective treatment of anaphylaxis. Advise the patient to go immediately to the nearest emergency room for further treatment of anaphylaxis. Additionally, injection into the buttock has been associated with gas gangrene. Cleansing with alcohol does not kill bacterial spores, and therefore, does not lower this risk.
- Do not inject into digits, hands or feet. Since epinephrine is a strong vasoconstrictor, accidental injection into the digits, hands or feet may result in loss of blood flow to the affected area. Advise the patient to go immediately to the nearest emergency room and to inform the healthcare provider in the emergency room of the location of the accidental injection. Treatment of such inadvertent administration should consist of vasodilation, in addition to further appropriate treatment of anaphylaxis [ see Adverse Reactions (6)].
- EpiPen and EpiPen Jr should only be injected into the anterolateral aspect of the thigh [see Dosage and Administration (2) and Patient Counseling Information (17)].
- Allergic Reactions Associated With Sulfite
- The presence of a sulfite in this product should not deter administration of the drug for treatment of serious allergic or other emergency situations even if the patient is sulfite-sensitive.
- Epinephrine is the preferred treatment for serious allergic reactions or other emergency situations even though this product contains sodium metabisulfite, a sulfite that may, in other products, cause allergic-type reactions including anaphylactic symptoms or life-threatening or less severe asthmatic episodes in certain susceptible persons.
- The alternatives to using epinephrine in a life-threatening situation may not be satisfactory.
- Disease Interactions
- Some patients may be at greater risk for developing adverse reactions after epinephrine administration. Despite these concerns, it should be recognized that the presence of these conditions is not a contraindication to epinephrine administration in an acute, life-threatening situation. Therefore, patients with these conditions, and/or any other person who might be in a position to administer EpiPen or EpiPen Jr to a patient experiencing anaphylaxis should be carefully instructed in regard to the circumstances under which epinephrine should be used.
- Patients with Heart Disease
- Epinephrine should be administered with caution to patients who have heart disease, including patients with cardiac arrhythmias, coronary artery or organic heart disease, or hypertension. In such patients, or in patients who are on drugs that may sensitize the heart to arrhythmias, epinephrine may precipitate or aggravate angina pectoris as well as produce ventricular arrhythmias.
- Other Patients and Diseases
- Epinephrine should be administered with caution to patients with hyperthyroidism, diabetes, elderly individuals, and pregnant women. Patients with Parkinson’s disease may notice a temporary worsening of symptoms.
Adverse Reactions
Clinical Trials Experience
- Due to the lack of randomized, controlled clinical trials of epinephrine for the treatment of anaphylaxis, the true incidence of adverse reactions associated with the systemic use of epinephrine is difficult to determine. Adverse reactions reported in observational trials, case reports, and studies are listed below.
- Common adverse reactions to systemically administered epinephrine include anxiety; apprehensiveness; restlessness; tremor; weakness; dizziness; sweating; palpitations; pallor; nausea and vomiting; headache; and/or respiratory difficulties. These symptoms occur in some persons receiving therapeutic doses of epinephrine, but are more likely to occur in patients with hypertension or hyperthyroidism [see Warnings and Precautions (5.4)].
- Arrhythmias, including fatal ventricular fibrillation, have been reported, particularly in patients with underlying cardiac disease or those receiving certain drugs [see Warnings and Precautions (5.4) and Drug Interactions (7)].
- Rapid rises in blood pressure have produced cerebral hemorrhage, particularly in elderly patients with cardiovascular disease [see Warnings and Precautions (5.4)].
- Angina may occur in patients with coronary artery disease [see Warnings and Precautions (5.4)].
- Accidental injection into the digits, hands or feet may result in loss of blood flow to the affected area [see Warnings and Precautions (5.2)].
- Adverse events experienced as a result of accidental injections may include increased heart rate, local reactions including injection site pallor, coldness and hypoesthesia or injury at the injection site resulting in bruising, bleeding, discoloration, erythema or skeletal injury.
- Injection into the buttock has resulted in cases of gas gangrene.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of EpiPen in the drug label.
Drug Interactions
- Patients who receive epinephrine while concomitantly taking cardiac glycosides, diuretics, or anti-arrhythmics should be observed carefully for the development of cardiac arrhythmias [see Warnings and Precautions (5.4)].
- The effects of epinephrine may be potentiated by tricyclic antidepressants, monoamine oxidase inhibitors, levothyroxine sodium, and certain antihistamines, notably chlorpheniramine, tripelennamine, and diphenhydramine.
- The cardiostimulating and bronchodilating effects of epinephrine are antagonized by beta- adrenergic blocking drugs, such as propranolol.
- The vasoconstricting and hypertensive effects of epinephrine are antagonized by alpha- adrenergic blocking drugs, such as phentolamine.
- Ergot alkaloids may also reverse the pressor effects of epinephrine.
Use in Specific Populations
Pregnancy
- Pregnancy Category C
- There are no adequate and well controlled studies of the acute effect of epinephrine in pregnant women.
- Epinephrine was teratogenic in rabbits, mice and hamsters. Epinephrine should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus (fetal anoxia, spontaneous abortion, or both).
- Epinephrine has been shown to have teratogenic effects when administered subcutaneously in rabbits at approximately 30 times the maximum recommended daily subcutaneous or intramuscular dose (on a mg/m2 basis at a maternal dose of 1.2 mg/kg/day for two to three days), in mice at approximately 7 times the maximum daily subcutaneous or intramuscular dose (on a mg/m2 basis at a maternal subcutaneous dose of 1 mg/kg/day for 10 days), and in hamsters at approximately 5 times the maximum recommended daily subcutaneous or intramuscular dose (on a mg/m2 basis at a maternal subcutaneous dose of 0.5 mg/kg/day for 4 days).
- These effects were not seen in mice at approximately 3 times the maximum recommended daily subcutaneous or intramuscular dose (on a mg/m2 basis at a subcutaneous maternal dose of 0.5 mg/kg/day for 10 days).
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of EpiPen in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of EpiPen during labor and delivery.
Nursing Mothers
- It is not known whether epinephrine is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when EpiPen is administered to a nursing woman.
Pediatric Use
- EpiPen or EpiPen Jr may be administered to pediatric patients at a dosage appropriate to body weight. Clinical experience with the use of epinephrine suggests that the adverse reactions seen in children are similar in nature and extent to those both expected and reported in adults. Since the doses of epinephrine delivered from EpiPen and EpiPen Jr are fixed, consider using other forms of injectable epinephrine if doses lower than 0.15 mg are deemed necessary.
Geriatic Use
- Clinical studies for the treatment of anaphylaxis have not been performed in subjects aged 65 and over to determine whether they respond differently from younger subjects. However, other reported clinical experience with use of epinephrine for the treatment of anaphylaxis has identified that geriatric patients may be particularly sensitive to the effects of epinephrine. Therefore, EpiPen should be administered with caution in elderly individuals, who may be at greater risk for developing adverse reactions after epinephrine administration.
Gender
There is no FDA guidance on the use of EpiPen with respect to specific gender populations.
Race
There is no FDA guidance on the use of EpiPen with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of EpiPen in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of EpiPen in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of EpiPen in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of EpiPen in patients who are immunocompromised.
Administration and Monitoring
Administration
- Intramuscular
Monitoring
There is limited information regarding Monitoring of EpiPen in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of EpiPen in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- Overdosage of epinephrine may produce extremely elevated arterial pressure, which may result in cerebrovascular hemorrhage, particularly in elderly patients. Overdosage may also result in pulmonary edema because of peripheral vascular constriction together with cardiac stimulation. Treatment consists of rapidly acting vasodilators or alpha-adrenergic blocking drugs and/or respiratory support.
- Epinephrine overdosage can also cause transient bradycardia followed by tachycardia, and these may be accompanied by potentially fatal cardiac arrhythmias. Premature ventricular contractions may appear within one minute after injection and may be followed by multifocal ventricular tachycardia (prefibrillation rhythm). Subsidence of the ventricular effects may be followed by atrial tachycardia and occasionally by atrioventricular block. Treatment of arrhythmias consists of administration of a beta-adrenergic blocking drug such as propranolol.
- Overdosage sometimes results in extreme pallor and coldness of the skin, metabolic acidosis, and kidney failure.
Management
- Suitable corrective measures must be taken in such situations.
Chronic Overdose
There is limited information regarding Chronic Overdose of EpiPen in the drug label.
Pharmacology
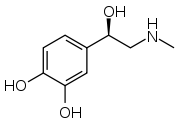
| |
EpiPen
| |
| Systematic (IUPAC) name | |
| (R)-4-(1-Hydroxy-2-(methylamino)ethyl)benzene-1,2-diol | |
| Identifiers | |
| CAS number | |
| ATC code | A01 B02BC09 (WHO) C01CA24 (WHO) R01AA14 (WHO) R03AA01 (WHO) S01EA01 (WHO) |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 183.204 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | adrenergic synapse (MAO and COMT) |
| Half life | 2 minutes |
| Excretion | Urine |
| Therapeutic considerations | |
| Pregnancy cat. |
C(US) |
| Legal status | |
| Routes | IV, IM, endotracheal, IC, Nasal Ophthalmic |
Mechanism of Action
Structure
Pharmacodynamics
There is limited information regarding Pharmacodynamics of EpiPen in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of EpiPen in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of EpiPen in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of EpiPen in the drug label.
How Supplied
Storage
There is limited information regarding EpiPen Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::EpiPen |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::EpiPen |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of EpiPen in the drug label.
Precautions with Alcohol
- Alcohol-EpiPen interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ®[1]
Look-Alike Drug Names
- A® — B®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Empty citation (help)
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=EpiPen |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=EpiPen |Label Name=EpiPen11.png
}}
{{#subobject:
|Label Page=EpiPen |Label Name=EpiPen11.png
}}
