Ependymoma pathophysiology
|
Ependymoma Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Ependymoma pathophysiology On the Web |
|
American Roentgen Ray Society Images of Ependymoma pathophysiology |
|
Risk calculators and risk factors for Ependymoma pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [2] Associate Editor(s)-in-Chief: Ahmad Al Maradni, M.D. [3]
Overview
On gross pathology, a well-encapsulated tumor which arises from the floor of the fourth ventricle, situated in the lower back portion of the brain is a characteristic finding of ependymoma. On microscopic histopathological analysis, perivascular pseudorosettes are characteristic findings of ependymoma. Development of ependymoma is the result of multiple genetic mutations (ERBB2, ERBB4, MMP2, MMP14, NOTCH1, and MEN1).[1]
Genetics
- One expression-defined group occurs primarily in young children and is characterized by a largely balanced genomic profile with an increased occurrence of chromosome 1q and proteins such as tenascin C and epidermal growth factor receptor.[2][3][4][5][6][7][8]
- The second expression-defined group occurs primarily in older children and adults and is characterized by a numerous cytogenetic abnormalities involving the whole chromosome or chromosomal arms.[9]
- Genes involved in ependymoma formation and progression are:[10]
- ERBB2
- ERBB4
- Human telomerase reverse transcriptase TERT
- KIT receptor tyrosine kinase and phospho-KIT receptor expression is associated with tumor progression
- MMP2 and MMP14 mutations appear to also play a role in tumor growth and progression in intracranial cases.
- NOTCH1 mutations have been found in approximately 8% of pediatric ependymomas
- MEN1 mutations are occasionally found in pediatric ependymomas.
- TPR and CHIBBY mutations have been identified in pediatric ependymomas.
- S100A6 and S100A4 on chromosome 1q have also been found to correspond to supratentorial tumor development and tumors occurring before the age of 3 years.
Associated Conditions
- The subependymal giant-cell astrocytoma, also called giant-cell glioma, is typically associated with tuberous sclerosis but can occur independently.
Pathology
- Ependymomas represent a relatively broad group of glial tumors which share common origin from differentiated ependymal cells lining the ventricles of the brain or the central canal of the spinal cord. They account for 5% of all neuroepithelial neoplasms.
- Ependymomas can occur anywhere, but certain location are typical. Common locations include:
- Floor of the fourth ventricle (common location in children)
- Spinal cord ependymoma
- Myxopapillary ependymoma (conus medullaris)
- Supratentorial ependymoma
- The subependymomas, variant of the ependymoma, arise in the fourth ventricle but may occur in the septum pellucidum and the cervical spinal cord.
Gross Pathology
- Ependymomas are well-encapsulated tumors which usually arise from the floor of the fourth ventricle.
-
Fourth ventricle ependymomas frequently extend out of the ventricle into the subarachnoid space.[11]
Microscopic Pathology
- Ependymomas are composed of cells with regular, round to oval nuclei. There is a variably dense fibrillary background.
- Tumor cells may form gland-like round or elongated structures that resemble the embryologic ependymal canal, with long, delicate processes extending into the lumen; more frequently present are perivascular pseudorosettes in which tumor cells are arranged around vessels with an intervening zone consisting of thin ependymal processes directed toward the wall of the vessel.[1]
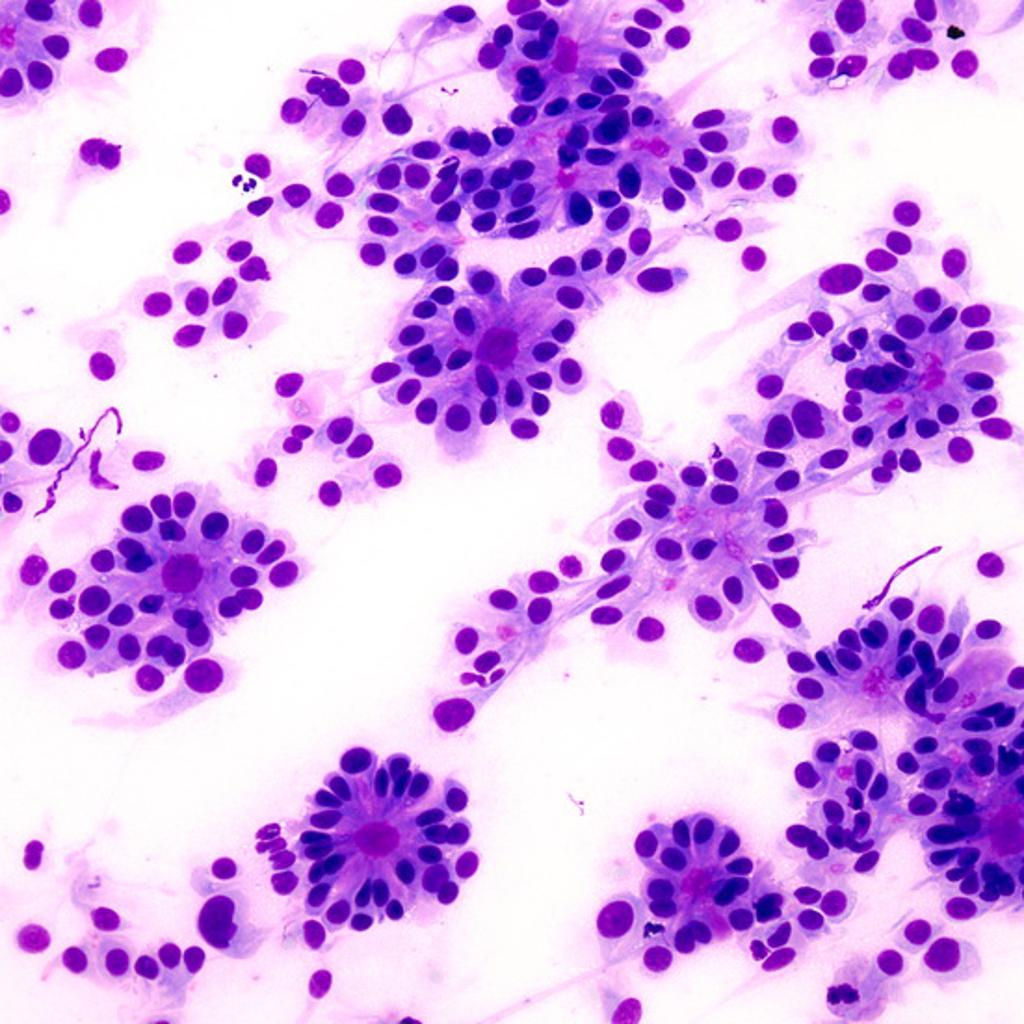
- Ependymal rosettes are rare but pathognomonic feature.
-
Micrograph of an ependymoma. H&E stain show pseudoressettes pattern of ependymal cells.[12]
-
True ependymal rosette consisting of tumor cells arranged around well-defined lumens forming gland-like structures.<ref name=radiopaedia> Image courtesy of Dr Dharam RamnaniRadiopaedia (original file
-
Higher magnification of one of the true ependymal rosettes showing a collar of cells around a central lumen forming a gland-like structure.[13]
References
- ↑ 1.0 1.1 Kumar, et al. (2005). The Central Nervous System. Pathologic Basis of Disease. 7th Edition. Philadelphia: Elsevier Saunders.
- ↑ Korshunov A, Golanov A, Timirgaz V (2000). "Immunohistochemical markers for intracranial ependymoma recurrence. An analysis of 88 cases". J Neurol Sci. 177 (1): 72–82. PMID 10967185.
- ↑ Mendrzyk F, Korshunov A, Benner A, Toedt G, Pfister S, Radlwimmer B; et al. (2006). "Identification of gains on 1q and epidermal growth factor receptor overexpression as independent prognostic markers in intracranial ependymoma". Clin Cancer Res. 12 (7 Pt 1): 2070–9. doi:10.1158/1078-0432.CCR-05-2363. PMID 16609018.
- ↑ Mohankumar KM, Currle DS, White E, Boulos N, Dapper J, Eden C; et al. (2015). "An in vivo screen identifies ependymoma oncogenes and tumor-suppressor genes". Nat Genet. 47 (8): 878–87. doi:10.1038/ng.3323. PMC 4520751. PMID 26075792.
- ↑ Abedalthagafi MS, Wu MP, Merrill PH, Du Z, Woo T, Sheu SH; et al. (2016). "Decreased FOXJ1 expression and its ciliogenesis programme in aggressive ependymoma and choroid plexus tumours". J Pathol. 238 (4): 584–97. doi:10.1002/path.4682. PMC 5364032. PMID 26690880.
- ↑ Georgescu MM, Yell P, Mobley BC, Shang P, Georgescu T, Wang SH; et al. (2015). "NHERF1/EBP50 is an organizer of polarity structures and a diagnostic marker in ependymoma". Acta Neuropathol Commun. 3: 11. doi:10.1186/s40478-015-0197-z. PMC 4352254. PMID 25775275.
- ↑ Andreiuolo F, Le Teuff G, Bayar MA, Kilday JP, Pietsch T, von Bueren AO; et al. (2017). "Integrating Tenascin-C protein expression and 1q25 copy number status in pediatric intracranial ependymoma prognostication: A new model for risk stratification". PLoS One. 12 (6): e0178351. doi:10.1371/journal.pone.0178351. PMC 5472261. PMID 28617804.
- ↑ Araki A, Chocholous M, Gojo J, Dorfer C, Czech T, Heinzl H; et al. (2016). "Chromosome 1q gain and tenascin-C expression are candidate markers to define different risk groups in pediatric posterior fossa ependymoma". Acta Neuropathol Commun. 4 (1): 88. doi:10.1186/s40478-016-0349-9. PMC 4994287. PMID 27550150.
- ↑ Wani K, Armstrong TS, Vera-Bolanos E, Raghunathan A, Ellison D, Gilbertson R; et al. (2012). "A prognostic gene expression signature in infratentorial ependymoma". Acta Neuropathol. 123 (5): 727–38. doi:10.1007/s00401-012-0941-4. PMC 4013829. PMID 22322993.
- ↑ Zakrzewska M, Fendler W, Zakrzewski K, Sikorska B, Grajkowska W, Dembowska-Bagińska B; et al. (2016). "Altered MicroRNA Expression Is Associated with Tumor Grade, Molecular Background and Outcome in Childhood Infratentorial Ependymoma". PLoS One. 11 (7): e0158464. doi:10.1371/journal.pone.0158464. PMC 4938415. PMID 27390862.
- ↑ EPENDYMOMA. http://librepathology.org/wiki/index.php/File:AFIP405713G-EPENDYMOMA.jpg 2015. URL Accessed on 10 6, 2015
- ↑ Ependymoma. https://en.wikipedia.org/wiki/Ependymoma#/media/File:Ependymoma_low_intermed_mag.jpg. URL Accessed on 10 6, 2015
- ↑ Image courtesy of Dr Dharam RamnaniRadiopaedia (original filehere). [1] CreativeCommons BY-SANC
![Fourth ventricle ependymomas frequently extend out of the ventricle into the subarachnoid space.[11]](/images/0/0f/Epyndemomas_gross.jpg)
![Micrograph of an ependymoma. H&E stain show pseudoressettes pattern of ependymal cells.[12]](/images/0/0a/532px-Ependymoma_low_intermed_mag.jpg)
![Higher magnification of one of the true ependymal rosettes showing a collar of cells around a central lumen forming a gland-like structure.[13]](/images/b/b6/Ependymoma-true-ependymal-rosettes123.jpg)