Chondrosarcoma diagnostic study of choice: Difference between revisions
Jump to navigation
Jump to search
(Created page with "__NOTOC__ {{Chondrosarcoma}} {{CMG}}; {{AE}} == Overview == == Diagnostic Study of Choice == === Study of choice === [Name of the investigation] is the gold standard test fo...") |
Farima Kahe (talk | contribs) No edit summary |
||
| (8 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
__NOTOC__ | __NOTOC__ | ||
{{Chondrosarcoma}} | {{Chondrosarcoma}} | ||
{{CMG}}; {{AE}} | {{CMG}}; {{AE}} {{Rohan}} | ||
== Overview == | == Overview == | ||
[[Biopsy]] is the gold standard test for the diagnosis of chondrosarcoma. Open [[biopsy]] is carried out for chondrosarcoma. The [[tumor]] is then staged based on Enneking system for chondrosarcoma. | |||
== Diagnostic Study of Choice == | == Diagnostic Study of Choice == | ||
=== Study of choice === | === Study of choice === | ||
[ | *[[Biopsy]] is the gold standard test for the diagnosis of chondrosarcoma. <ref>{{cite book | last = Peabody | first = Terrance | title = Orthopaedic oncology : primary and metastatic tumors of the skeletal system | publisher = Springer | location = Cham | year = 2014 | isbn = 9783319073224 }}</ref><ref>{{cite book | last = Czerniak | first = Bogdan | title = Dorfman and Czerniak's bone tumors | publisher = Elsevier/Saunders | location = Philadelphia, PA | year = 2016 | isbn = 9780323023962 }}</ref> | ||
'''Prerequisites for a Biopsy''' | |||
*[[Complete blood count|CBC]], [[Platelet|platelets]] and [[coagulation studies]] are required. | |||
*Cross-sectional [[imaging]] to evaluate local anatomy such [[Computed tomography|CT scan]] and [[Magnetic resonance imaging|MRI]]. | |||
*Treatment center carrying out [[biopsy]] must be capable of proper [[diagnosis]] and treatment. | |||
*The surgeon who performs [[biopsy]] should preferably be the one who is later going to do the final excision. | |||
'''Technique''' | |||
* | *Open | ||
* | *Closed | ||
===Open Technique=== | |||
*The [[tumor]] is surgically exposed and [[biopsy]] of the tumor is taken. | |||
{| align="right" | |||
| | |||
[[File:Biopsy.jpg|300px|thumb| Open Biopsy.[https://commons.wikimedia.org/wiki/File:Brain_biopsy_under_stereotaxy.jpg Source: Case courtesy of Dake~commonswiki, via Wikimedia Commons]]] | |||
|} | |||
===Types=== | |||
===Incisional biopsy=== | |||
*A small surgical incision carefully placed to access [[tumor]] without contamination of critical structures. | |||
===Excisional biopsy=== | |||
* | *it is done for small, superficial [[soft tissue]] masses. | ||
'''Incision''' | |||
*Longitudinal [[incision]] in the [[extremities]] is taken. | |||
*It should allow for [[extension]] of the [[incision]] for definitive management. | |||
[ | '''Approach''' | ||
*Never expose [[Neurovascular bundle|neurovascular]] structures. | |||
*During the [[biopsy]], all tissue exposed is considered contaminated with [[tumor]]. | |||
*Meticulous [[hemostasis]] to be carried out. | |||
*Post-surgery [[Hematoma|hematoma']]<nowiki/>s are considered contaminated with tumor. | |||
*Always deflate the [[tourniquet]] prior to wound closure. | |||
'''Biopsy''' | |||
*Perform through the involved [[Compartment (anatomy)|compartment]] of the [[tumor]]. | |||
*For [[bone]] lesions with a [[soft tissue]] mass, perform the [[biopsy]] using the soft tissue mass. | |||
''''Closure''' | |||
*If [[Drain (surgery)|drain]] is kept, remove the [[Drain (surgery)|drain]] out of the skin in line with surgical [[incision]]. | |||
*This helps in excising the drain site with definitive surgical extensile [[incision]]. | |||
===Closed Technique=== | |||
===Types=== | |||
===Fine Needle Aspiration (FNA)=== | |||
*It provides [[Cytological|cytologic]] specimen. | |||
*It is the most commonly used for [[carcinoma]]. | |||
*It is usually not preferred for [[sarcoma]]. | |||
{| align="right" | |||
| | |||
[[File:Needle biopsy.jpg|300px|thumb| Needle Biopsy.[https://upload.wikimedia.org/wikipedia/commons/0/02/Needle_biopsy.jpg Source: Case courtesy of Linda Bartlett (photographer) [Public domain], via Wikimedia Commons]]] | |||
|} | |||
===Core biopsy (Tru-cut Biopsy)=== | |||
*It allows for [[tumor]] structural examination. | |||
*It allows evaluation of both the [[Cytological|cytologic]] and [[stromal]] elements of the [[tumor]]. | |||
*It is frequently used for [[sarcoma]]. | |||
===== Sequence of Diagnostic Studies ===== | |||
The various investigations must be performed in the following order: | |||
*[[X-rays|X-ray]] | |||
*[[MRI]] | |||
*[[Biopsy]] | |||
===Staging=== | |||
===Enneking (MSTS) Staging System=== | |||
*The Enneking surgical staging system (also known as the MSTS system) for malignant [[Musculoskeletal system|musculoskeletal]] [[Tumor|tumors]] based on [[Histology|histological]] and [[radiographic]] characteristics of the [[tumor]] host margin.<ref name="pmid20333492">{{cite journal| author=Jawad MU, Scully SP| title=In brief: classifications in brief: enneking classification: benign and malignant tumors of the musculoskeletal system. | journal=Clin Orthop Relat Res | year= 2010 | volume= 468 | issue= 7 | pages= 2000-2 | pmid=20333492 | doi=10.1007/s11999-010-1315-7 | pmc=2882012 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=20333492 }} </ref><ref>{{cite book | last = Peabody | first = Terrance | title = Orthopaedic oncology : primary and metastatic tumors of the skeletal system | publisher = Springer | location = Cham | year = 2014 | isbn = 9783319073224 }}</ref> | |||
*It is widely accepted and routinely used staging system. | |||
{| style="border: 0px; font-size: 90%; margin: 3px; width: 1000px" align="center" | |||
| valign="top" | | |||
|- | |||
! style="background: #4479BA; width: 200px;" | {{fontcolor|#FFF|Stages}} | |||
! style="background: #4479BA; width: 300px;" |{{fontcolor|#FFF|Grade}} | |||
! style="background: #4479BA; width: 300px;" |{{fontcolor|#FFF|Site}} | |||
! style="background: #4479BA; width: 300px;" |{{fontcolor|#FFF|Metastasis}} | |||
|- | |||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;" |IA | |||
| style="padding: 5px 5px; background: #F5F5F5; font-weight: bold; text-align:center;" | G1: Low grade | |||
| style="padding: 5px 5px; background: #F5F5F5; font-weight: bold; text-align:center;" | T1: Intracompartmental | |||
==== | | style="padding: 5px 5px; background: #F5F5F5; font-weight: bold; text-align:center;" | M0: No metastasis | ||
|- | |||
| | | style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;" |IB | ||
| style="padding: 5px 5px; background: #F5F5F5; font-weight: bold; text-align:center;" | G1: Low grade | |||
| style="padding: 5px 5px; background: #F5F5F5; font-weight: bold; text-align:center;" | T2: Extracompartmental | |||
| style="padding: 5px 5px; background: #F5F5F5; font-weight: bold; text-align:center;" | M0: No metastasis | |||
|- | |||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;" |IIA | |||
| style="padding: 5px 5px; background: #F5F5F5; font-weight: bold; text-align:center;" | G2: High grade | |||
| style="padding: 5px 5px; background: #F5F5F5; font-weight: bold; text-align:center;" | T1: Intracompartmental | |||
| style="padding: 5px 5px; background: #F5F5F5; font-weight: bold; text-align:center;" | M0: No metastasis | |||
|- | |- | ||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;" |IIB | |||
| style="background: # | | style="padding: 5px 5px; background: #F5F5F5; font-weight: bold; text-align:center;" | G2: High grade | ||
| style="background: # | | style="padding: 5px 5px; background: #F5F5F5; font-weight: bold; text-align:center;" | T2: Extracompartmental | ||
| style="padding: 5px 5px; background: #F5F5F5; font-weight: bold; text-align:center;" | M0: No metastasis | |||
|- | |- | ||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;" |III | |||
| style="background: # | | style="padding: 5px 5px; background: #F5F5F5; font-weight: bold; text-align:center;" | G1 or G2 | ||
| style="background: # | | style="padding: 5px 5px; background: #F5F5F5; font-weight: bold; text-align:center;" | T1 or T2 | ||
| style="padding: 5px 5px; background: #F5F5F5; font-weight: bold; text-align:center;" | M1: Regional or distant metastasis | |||
|} | |} | ||
==References== | ==References== | ||
| Line 130: | Line 118: | ||
{{WH}} | {{WH}} | ||
{{WS}} | {{WS}} | ||
[[Category: Orthopedics]] | |||
[[Category: Oncology]] | |||
[[Category: Up-To-Date]] | |||
Latest revision as of 18:11, 14 March 2019
|
Chondrosarcoma Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Chondrosarcoma diagnostic study of choice On the Web |
|
American Roentgen Ray Society Images of Chondrosarcoma diagnostic study of choice |
|
Risk calculators and risk factors for Chondrosarcoma diagnostic study of choice |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Rohan A. Bhimani, M.B.B.S., D.N.B., M.Ch.[2]
Overview
Biopsy is the gold standard test for the diagnosis of chondrosarcoma. Open biopsy is carried out for chondrosarcoma. The tumor is then staged based on Enneking system for chondrosarcoma.
Diagnostic Study of Choice
Study of choice
Prerequisites for a Biopsy
- CBC, platelets and coagulation studies are required.
- Cross-sectional imaging to evaluate local anatomy such CT scan and MRI.
- Treatment center carrying out biopsy must be capable of proper diagnosis and treatment.
- The surgeon who performs biopsy should preferably be the one who is later going to do the final excision.
Technique
- Open
- Closed
Open Technique
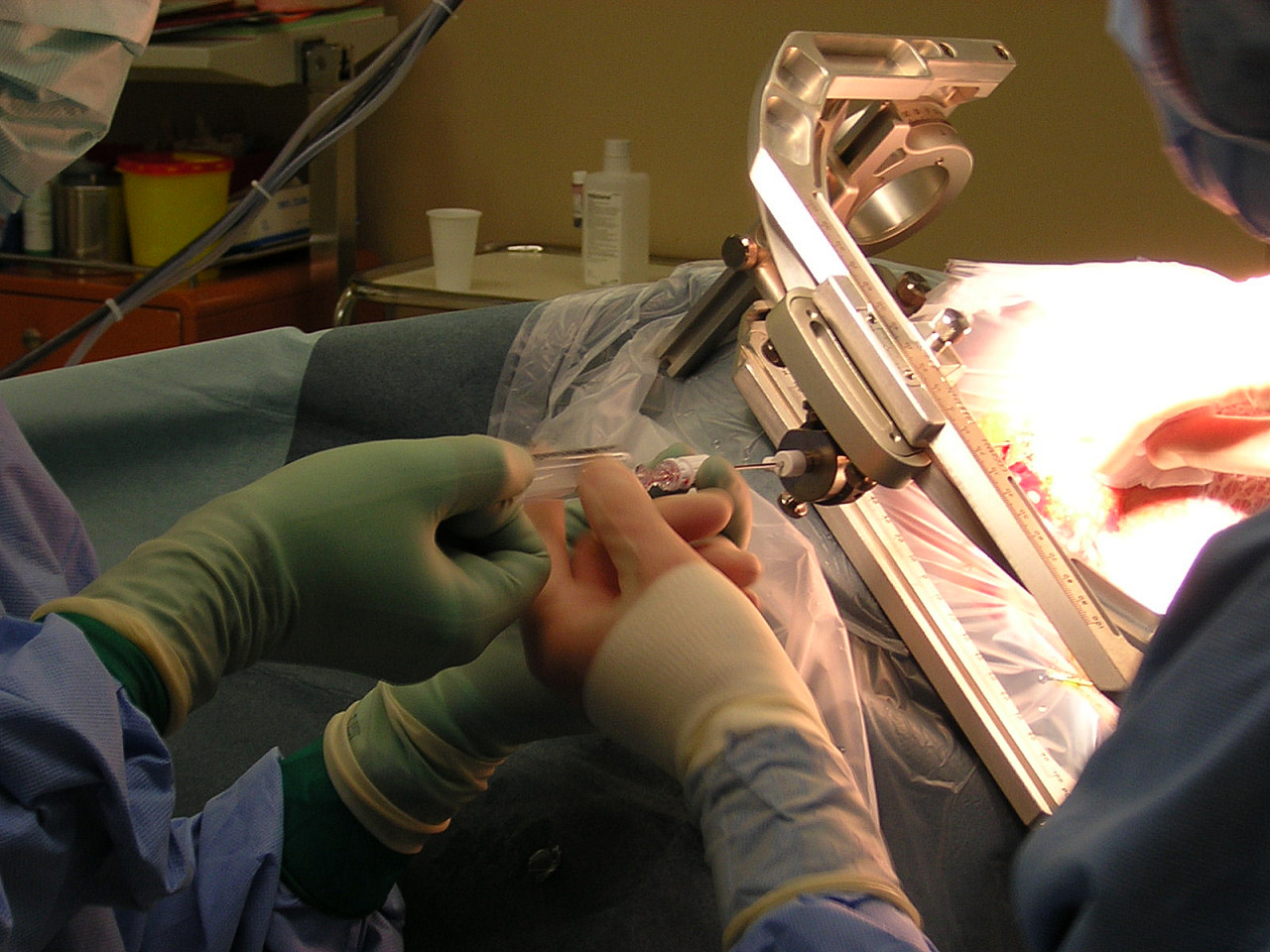 |
Types
Incisional biopsy
- A small surgical incision carefully placed to access tumor without contamination of critical structures.
Excisional biopsy
- it is done for small, superficial soft tissue masses.
Incision
- Longitudinal incision in the extremities is taken.
- It should allow for extension of the incision for definitive management.
Approach
- Never expose neurovascular structures.
- During the biopsy, all tissue exposed is considered contaminated with tumor.
- Meticulous hemostasis to be carried out.
- Post-surgery hematoma's are considered contaminated with tumor.
- Always deflate the tourniquet prior to wound closure.
Biopsy
- Perform through the involved compartment of the tumor.
- For bone lesions with a soft tissue mass, perform the biopsy using the soft tissue mass.
'Closure
- If drain is kept, remove the drain out of the skin in line with surgical incision.
- This helps in excising the drain site with definitive surgical extensile incision.
Closed Technique
Types
Fine Needle Aspiration (FNA)
- It provides cytologic specimen.
- It is the most commonly used for carcinoma.
- It is usually not preferred for sarcoma.
 |
Core biopsy (Tru-cut Biopsy)
- It allows for tumor structural examination.
- It allows evaluation of both the cytologic and stromal elements of the tumor.
- It is frequently used for sarcoma.
Sequence of Diagnostic Studies
The various investigations must be performed in the following order:
Staging
Enneking (MSTS) Staging System
- The Enneking surgical staging system (also known as the MSTS system) for malignant musculoskeletal tumors based on histological and radiographic characteristics of the tumor host margin.[3][4]
- It is widely accepted and routinely used staging system.
| Stages | Grade | Site | Metastasis |
|---|---|---|---|
| IA | G1: Low grade | T1: Intracompartmental | M0: No metastasis |
| IB | G1: Low grade | T2: Extracompartmental | M0: No metastasis |
| IIA | G2: High grade | T1: Intracompartmental | M0: No metastasis |
| IIB | G2: High grade | T2: Extracompartmental | M0: No metastasis |
| III | G1 or G2 | T1 or T2 | M1: Regional or distant metastasis |
References
- ↑ Peabody, Terrance (2014). Orthopaedic oncology : primary and metastatic tumors of the skeletal system. Cham: Springer. ISBN 9783319073224.
- ↑ Czerniak, Bogdan (2016). Dorfman and Czerniak's bone tumors. Philadelphia, PA: Elsevier/Saunders. ISBN 9780323023962.
- ↑ Jawad MU, Scully SP (2010). "In brief: classifications in brief: enneking classification: benign and malignant tumors of the musculoskeletal system". Clin Orthop Relat Res. 468 (7): 2000–2. doi:10.1007/s11999-010-1315-7. PMC 2882012. PMID 20333492.
- ↑ Peabody, Terrance (2014). Orthopaedic oncology : primary and metastatic tumors of the skeletal system. Cham: Springer. ISBN 9783319073224.