Canagliflozin: Difference between revisions
No edit summary |
No edit summary |
||
| Line 13: | Line 13: | ||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Canagliflozin in pediatric patients. | |offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Canagliflozin in pediatric patients. | ||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Canagliflozin in pediatric patients. | |offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Canagliflozin in pediatric patients. | ||
|contraindications=*History of a serious hypersensitivity reaction to INVOKANA | |||
*Severe renal impairment (eGFR less than 30 mL/min/1.73 m2), end stage renal disease or patients on dialysis. | |||
|warnings======Hypotension===== | |warnings======Hypotension===== | ||
INVOKANA causes [[intravascular volume]] contraction. Symptomatic [[hypotension]] can occur after initiating INVOKANA, particularly in patients with [[impaired renal function]] ([[eGFR]] less than 60 mL/min/1.73 m2), elderly patients, patients on either [[diuretics]] or medications that interfere with the [[renin-angiotensin-aldosterone system]] (e.g., [[angiotensin-converting-enzyme]] [[ACE]] inhibitors, [[angiotensin receptor blockers]] [[ARBs]]), or patients with low [[systolic blood pressure]]. Before initiating INVOKANA in patients with one or more of these characteristics, volume status should be assessed and corrected. Monitor for signs and symptoms after initiating therapy. | INVOKANA causes [[intravascular volume]] contraction. Symptomatic [[hypotension]] can occur after initiating INVOKANA, particularly in patients with [[impaired renal function]] ([[eGFR]] less than 60 mL/min/1.73 m2), elderly patients, patients on either [[diuretics]] or medications that interfere with the [[renin-angiotensin-aldosterone system]] (e.g., [[angiotensin-converting-enzyme]] [[ACE]] inhibitors, [[angiotensin receptor blockers]] [[ARBs]]), or patients with low [[systolic blood pressure]]. Before initiating INVOKANA in patients with one or more of these characteristics, volume status should be assessed and corrected. Monitor for signs and symptoms after initiating therapy. | ||
| Line 49: | Line 51: | ||
=====Interference with 1,5-anhydroglucitol (1,5-AG) Assay===== | =====Interference with 1,5-anhydroglucitol (1,5-AG) Assay===== | ||
Monitoring glycemic control with 1,5-AG assay is not recommended as measurements of 1,5-AG are unreliable in assessing glycemic control in patients taking SGLT2 inhibitors. Use alternative methods to monitor glycemic control. | Monitoring glycemic control with 1,5-AG assay is not recommended as measurements of 1,5-AG are unreliable in assessing glycemic control in patients taking SGLT2 inhibitors. Use alternative methods to monitor glycemic control. | ||
|drugBox={{drugbox2 | |||
| Verifiedfields = changed | |||
| Watchedfields = changed | |||
| tradename = Invokana | |||
| Drugs.com = {{Drugs.com|parent|invokana}} | |||
| UNII_Ref = {{fdacite|changed|FDA}} | |||
| UNII = 6S49DGR869 | |||
| verifiedrevid = 416773181 | |||
| IUPAC_name = (2''S'',3''R'',4''R'',5''S'',6''R'')-2-{3-[5-[4-Fluoro-phenyl)-thiophen-2-ylmethyl]-4-methyl-phenyl}-6-hydroxymethyl-tetrahydro-pyran-3,4,5-triol | |||
| synonyms = JNJ-28431754; TA-7284; (1''S'')-1,5-anhydro-1-''C''-[3-<nowiki>[[</nowiki>5-(4-fluorophenyl)-2-thienyl]methyl]-4-methylphenyl]-<small>D</small>-glucitol | |||
| image = Anag Strcutture.png | |||
| alt = 300px | |||
| CAS_number_Ref = {{cascite|correct|??}} | |||
| CAS_number = 842133-18-0 | |||
| ATCvet = | |||
| ATC_prefix = A10 | |||
| ATC_suffix = BX11 | |||
| PubChem = 24812758 | |||
| ChEMBL_Ref = {{ebicite|changed|EBI}} | |||
| ChEMBL = 2103841 | |||
| ChemSpiderID_Ref = {{chemspidercite|changed|chemspider}} | |||
| ChemSpiderID = 26333259 | |||
| smiles = Cc1ccc(cc1Cc2ccc(s2)c3ccc(cc3)F)[C@H]4[C@@H]([C@H]([C@@H]([C@H](O4)CO)O)O)O | |||
| InChI = 1/C24H25FO5S/c1-13-2-3-15(24-23(29)22(28)21(27)19(12-26)30-24)10-16(13)11-18-8-9-20(31-18)14-4-6-17(25)7-5-14/h2-10,19,21-24,26-29H,11-12H2,1H3/t19-,21-,22+,23-,24+/m1/s1 | |||
| InChIKey = XTNGUQKDFGDXSJ-ZXGKGEBGBZ | |||
| StdInChI_Ref = {{stdinchicite|changed|chemspider}} | |||
| StdInChI = 1S/C24H25FO5S/c1-13-2-3-15(24-23(29)22(28)21(27)19(12-26)30-24)10-16(13)11-18-8-9-20(31-18)14-4-6-17(25)7-5-14/h2-10,19,21-24,26-29H,11-12H2,1H3/t19-,21-,22+,23-,24+/m1/s1 | |||
| StdInChIKey_Ref = {{stdinchicite|changed|chemspider}} | |||
| StdInChIKey = XTNGUQKDFGDXSJ-ZXGKGEBGSA-N | |||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| DrugBank = | |||
| ChEBI_Ref = {{ebicite|changed|EBI}} | |||
| ChEBI = 73274 | |||
| C=24 | H=25 | F=1 | O=5 | S=1 | |||
| molecular_weight = 444.52 g/mol | |||
| bioavailability = 65% | |||
| protein_bound = 99% | |||
| metabolism = [[Liver|Hepatic]] [[glucuronidation]] | |||
| elimination_half-life = 11.8 (10–13) hours | |||
| excretion = Fecal and 33% renal | |||
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> | |||
| pregnancy_US = C | |||
| pregnancy_category= | |||
| legal_AU = <!-- S2, S3, S4, S5, S6, S7, S8, S9 or Unscheduled--> | |||
| legal_CA = <!-- Schedule I, II, III, IV, V, VI, VII, VIII --> | |||
| legal_UK = <!-- GSL, P, POM, CD, or Class A, B, C --> | |||
| legal_US = Rx-only | |||
| legal_status = | |||
| routes_of_administration = Oral | |||
}} | |||
|mechAction=Sodium-glucose co-transporter 2 (SGLT2), expressed in the proximal renal tubules, is responsible for the majority of the reabsorption of filtered glucose from the tubular lumen. Canagliflozin is an inhibitor of SGLT2. By inhibiting SGLT2, canagliflozin reduces reabsorption of filtered glucose and lowers the renal threshold for glucose (RTG), and thereby increases urinary glucose excretion. | |||
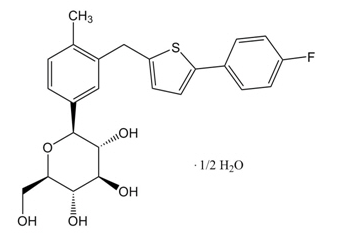
|structure=Canagliflozin, the active ingredient of INVOKANA, is chemically known as (1S)-1,5-anhydro-1-[3-[[5-(4-fluorophenyl)-2-thienyl]methyl]-4-methylphenyl]-D-glucitol hemihydrate and its molecular formula and weight are C24H25FO5S∙1/2 H2O and 453.53, respectively. The structural formula for canagliflozin : | |||
[[file:Anag Strcutture.png|none|400px]] | |||
|PD=Following single and multiple oral doses of canagliflozin to patients with type 2 diabetes, dose-dependent decreases in the renal threshold for glucose (RTG) and increases in urinary glucose excretion were observed. From a starting value of RTG of approximately 240 mg/dL, canagliflozin at 100 mg and 300 mg once daily suppressed RTG throughout the 24-hour period. Maximal suppression of mean RTG over the 24-hour period was seen with the 300 mg daily dose to approximately 70 to 90 mg/dL in patients with type 2 diabetes in Phase 1 studies. In patients with type 2 diabetes given 100 mg to 300 mg once daily over a 16-day dosing period, reductions in RTG and increases in urinary glucose excretion were observed over the dosing period. In this study, plasma glucose declined in a dose-dependent fashion within the first day of dosing. In single-dose studies in healthy and type 2 diabetic subjects, treatment with canagliflozin 300 mg before a mixed-meal delayed intestinal glucose absorption and reduced postprandial glucose. | |||
======Cardiac Electrophysiology====== | |||
|alcohol=Alcohol-Canagliflozin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=Alcohol-Canagliflozin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
}} | }} | ||
Revision as of 20:51, 19 January 2015
{{DrugProjectFormSinglePage |authorTag=Alberto Plate [1] |genericName=Canagliflozin |aOrAn=a |drugClass=Endocrine-Metabolic Agent and Sodium Glucose Co-Transporter 2 Inhibitor |indicationType=treatment |indication=diabetes mellitus type 2 |adverseReactions=Micturition frequency and polyuria, Urinary tract infectious disease, Mycosis of the Female genital |blackBoxWarningTitle=TITLE |blackBoxWarningBody=Condition Name: (Content) |offLabelAdultGuideSupport=There is limited information regarding Off-Label Guideline-Supported Use of Canagliflozin in adult patients. |offLabelAdultNoGuideSupport=There is limited information regarding Off-Label Non–Guideline-Supported Use of Canagliflozin in adult patients. |offLabelPedGuideSupport=There is limited information regarding Off-Label Guideline-Supported Use of Canagliflozin in pediatric patients. |offLabelPedNoGuideSupport=There is limited information regarding Off-Label Non–Guideline-Supported Use of Canagliflozin in pediatric patients. |contraindications=*History of a serious hypersensitivity reaction to INVOKANA
- Severe renal impairment (eGFR less than 30 mL/min/1.73 m2), end stage renal disease or patients on dialysis.
|warnings======Hypotension===== INVOKANA causes intravascular volume contraction. Symptomatic hypotension can occur after initiating INVOKANA, particularly in patients with impaired renal function (eGFR less than 60 mL/min/1.73 m2), elderly patients, patients on either diuretics or medications that interfere with the renin-angiotensin-aldosterone system (e.g., angiotensin-converting-enzyme ACE inhibitors, angiotensin receptor blockers ARBs), or patients with low systolic blood pressure. Before initiating INVOKANA in patients with one or more of these characteristics, volume status should be assessed and corrected. Monitor for signs and symptoms after initiating therapy.
Impairment in Renal Function
INVOKANA increases serum creatinine and decreases eGFR. Patients with hypovolemia may be more susceptible to these changes. Renal function abnormalities can occur after initiating INVOKANA. More frequent renal function monitoring is recommended in patients with an eGFR below 60 mL/min/1.73 m2.
Hyperkalemia
INVOKANA can lead to hyperkalemia. Patients with moderate renal impairment who are taking medications that interfere with potassium excretion, such as potassium-sparing diuretics, or medications that interfere with the renin-angiotensin-aldosterone system are more likely to develop hyperkalemia.
Monitor serum potassium levels periodically after initiating INVOKANA in patients with impaired renal function and in patients predisposed to hyperkalemia due to medications or other medical conditions.
Hypoglycemia with Concomitant Use with Insulin and Insulin Secretagogues
Insulin and insulin secretagogues are known to cause hypoglycemia. INVOKANA can increase the risk of hypoglycemia when combined with insulin or an insulin secretagogue. Therefore, a lower dose of insulin or insulin secretagogue may be required to minimize the risk of hypoglycemia when used in combination with INVOKANA.
Genital Mycotic Infections
INVOKANA increases the risk of genital mycotic infections. Patients with a history of genital mycotic infections and uncircumcised males were more likely to develop genital mycotic infections. Monitor and treat appropriately.
5.6 Hypersensitivity Reactions
Hypersensitivity reactions (e.g., generalized urticaria), some serious, were reported with INVOKANA treatment; these reactions generally occurred within hours to days after initiating INVOKANA. If hypersensitivity reactions occur, discontinue use of INVOKANA; treat per standard of care and monitor until signs and symptoms resolve [see CONTRAINDICATIONS (4) and ADVERSE REACTIONS (6.1)].
Increases in Low-Density Lipoprotein (LDL-C)
Dose-related increases in LDL-C occur with INVOKANA. Monitor LDL-C and treat per standard of care after initiating INVOKANA.
Macrovascular Outcomes
There have been no clinical studies establishing conclusive evidence of macrovascular risk reduction with INVOKANA or any other antidiabetic drug. |drugInteractions======UGT Enzyme Inducers===== Rifampin: Co-administration of canagliflozin with rifampin, a nonselective inducer of several UGT enzymes, including UGT1A9, UGT2B4, decreased canagliflozin area under the curve (AUC) by 51%. This decrease in exposure to canagliflozin may decrease efficacy. If an inducer of these UGTs (e.g., rifampin, phenytoin, phenobarbital, ritonavir) must be co-administered with INVOKANA (canagliflozin), consider increasing the dose to 300 mg once daily if patients are currently tolerating INVOKANA 100 mg once daily, have an eGFR greater than 60 mL/min/1.73 m2, and require additional glycemic control. Consider other antihyperglycemic therapy in patients with an eGFR of 45 to less than 60 mL/min/1.73 m2 receiving concurrent therapy with a UGT inducer and require additional glycemic control.
Digoxin
There was an increase in the AUC and mean peak drug concentration (Cmax) of digoxin (20% and 36%, respectively) when co-administered with INVOKANA 300 mg. Patients taking INVOKANA with concomitant digoxin should be monitored appropriately.
Positive Urine Glucose Test
Monitoring glycemic control with urine glucose tests is not recommended in patients taking SGLT2 inhibitors as SGLT2 inhibitors increase urinary glucose excretion and will lead to positive urine glucose tests. Use alternative methods to monitor glycemic control.
Interference with 1,5-anhydroglucitol (1,5-AG) Assay
Monitoring glycemic control with 1,5-AG assay is not recommended as measurements of 1,5-AG are unreliable in assessing glycemic control in patients taking SGLT2 inhibitors. Use alternative methods to monitor glycemic control. |drugBox=
|mechAction=Sodium-glucose co-transporter 2 (SGLT2), expressed in the proximal renal tubules, is responsible for the majority of the reabsorption of filtered glucose from the tubular lumen. Canagliflozin is an inhibitor of SGLT2. By inhibiting SGLT2, canagliflozin reduces reabsorption of filtered glucose and lowers the renal threshold for glucose (RTG), and thereby increases urinary glucose excretion. |structure=Canagliflozin, the active ingredient of INVOKANA, is chemically known as (1S)-1,5-anhydro-1-[3-[[5-(4-fluorophenyl)-2-thienyl]methyl]-4-methylphenyl]-D-glucitol hemihydrate and its molecular formula and weight are C24H25FO5S∙1/2 H2O and 453.53, respectively. The structural formula for canagliflozin :
|PD=Following single and multiple oral doses of canagliflozin to patients with type 2 diabetes, dose-dependent decreases in the renal threshold for glucose (RTG) and increases in urinary glucose excretion were observed. From a starting value of RTG of approximately 240 mg/dL, canagliflozin at 100 mg and 300 mg once daily suppressed RTG throughout the 24-hour period. Maximal suppression of mean RTG over the 24-hour period was seen with the 300 mg daily dose to approximately 70 to 90 mg/dL in patients with type 2 diabetes in Phase 1 studies. In patients with type 2 diabetes given 100 mg to 300 mg once daily over a 16-day dosing period, reductions in RTG and increases in urinary glucose excretion were observed over the dosing period. In this study, plasma glucose declined in a dose-dependent fashion within the first day of dosing. In single-dose studies in healthy and type 2 diabetic subjects, treatment with canagliflozin 300 mg before a mixed-meal delayed intestinal glucose absorption and reduced postprandial glucose.
Cardiac Electrophysiology
|alcohol=Alcohol-Canagliflozin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. }}