Bococizumab
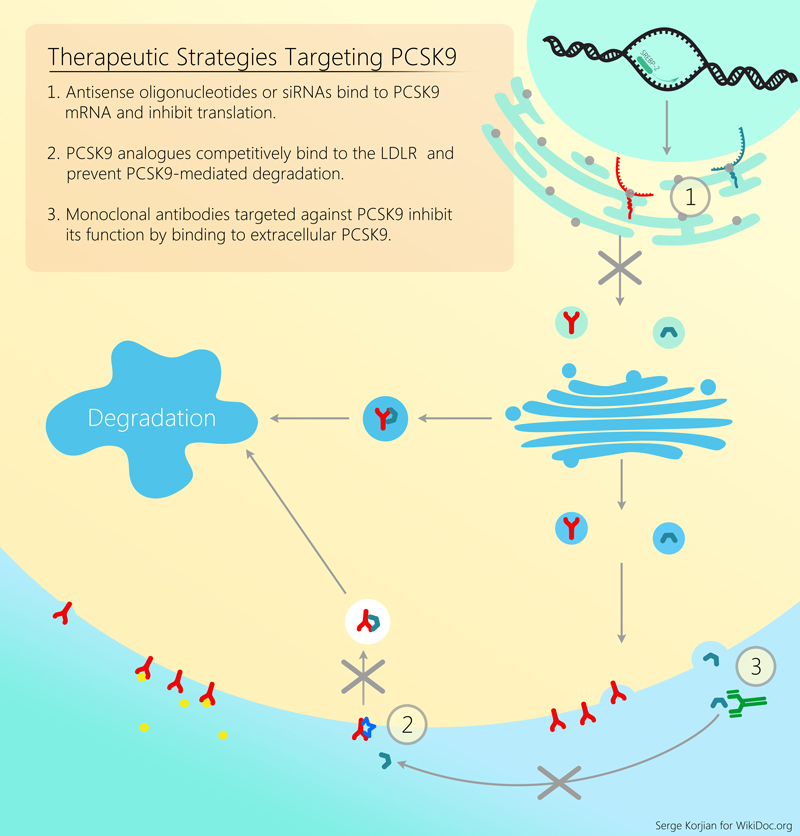
For a review of all PCSK9 inhibitors please click here
|
WikiDoc Resources for Bococizumab |
|
Articles |
|---|
|
Most recent articles on Bococizumab Most cited articles on Bococizumab |
|
Media |
|
Powerpoint slides on Bococizumab |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Bococizumab at Clinical Trials.gov Clinical Trials on Bococizumab at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Bococizumab
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Bococizumab Discussion groups on Bococizumab Patient Handouts on Bococizumab Directions to Hospitals Treating Bococizumab Risk calculators and risk factors for Bococizumab
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Bococizumab |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Bococizumab (PF-04950615; RN316) is a humanized monoclonal antibody that binds proprotein convertase subtilisin/kexin type 9 and is an investigational agent for the reduction of LDL-C levels in patients with hypercholesterolemia.
Properties
Major Trials
Phase II Trials
A 24 week, randomized, placebo-controlled, dose-ranging phase IIB trial was conducted in 354 patients to examine two different doses of bococizumab: a twice monthly dose of either 50, 100 or 150 mg; and a once monthly dose of either 200 or 300 mg. A dose reduction was made at week 6 for the twice monthly and at week 8 for the once monthly regimen in patients with LDL-C ≤25 mg/dL. The primary efficacy endpoint was the placebo-adjusted change from baseline in LDL-C at week 12. The study met its primary endpoint across all doses with a safety and tolerability profile equivalent to placebo.
| Dosing regimens | Mean change from baseline in LDL-C at Week 12 (placebo-adjusted) | Maximum mean change from baseline in LDL-C (placebo-adjusted) |
| 150 mg twice monthly | -53.4 mg/dL | -66.9 mg/dL (week 8) |
| 300 mg once monthly | -44.9 mg/dL | -54.9 mg/dL (week 4) |
Phase III Trials
SPIRE-HF
The SPIRE-HF trial aims to compare the effect of bococizumab in combination with a statin vs. placebo in combination with a statin on LDL-C level at 12 weeks in patients with heterozygous familial hypercholesterolemia. The trial is currently recruiting patients and is expected to be completed in January 2016.[2]
SPIRE-HR & SPIRE-LDL
The SPIRE-HR and SPIRE-LDL trial aims to compare the effect of bococizumab in combination with a statin vs. placebo in combination with a statin on LDL-C level at 12 weeks in patients with hypercholesterolemia and high cardiovascular risk. These trials are currently recruiting patients and are expected to be completed by January 2016.[2]
SPIRE-1
The SPIRE-1 trial aims to evaluate the effect of bococizumab vs. placebo (without specification regarding statin therapy) on the reduction of major cardiovascular events at 5 years, including cardiovascular death, myocardial infarction, stroke, and unstable angina requiring urgent revascularization. The trial will only include patients with LDL-C ≥ 70 mg/dL (1.8 mmol/L) and < 100 mg/dL (2.6 mmol/L). The trial is currently recruiting patients and is expected to be completed in August 2017.[2]
SPIRE-2
The SPIRE-1 trial aims to investigate the effect of bococizumab vs. placebo (without specification regarding statin therapy) on the reduction of major cardiovascular events at 5 years, including cardiovascular death, myocardial infarction, stroke, and unstable angina requiring urgent revascularization. The trial will only include patients with LDL-C ≥ 100 mg/dL (2.6 mmol/L) compared to the SPIRE-1 trial. The trial is currently recruiting patients and is expected to be completed in August 2017.[2]
References
- ↑ Urban, D.; Pöss, J.; Böhm, M.; Laufs, U. (2013). "Targeting the proprotein convertase subtilisin/kexin type 9 for the treatment of dyslipidemia and atherosclerosis". J Am Coll Cardiol. 62 (16): 1401–8. doi:10.1016/j.jacc.2013.07.056. PMID 23973703. Unknown parameter
|month=ignored (help) - ↑ 2.0 2.1 2.2 2.3 Dadu RT, Ballantyne CM (2014). "Lipid lowering with PCSK9 inhibitors". Nat Rev Cardiol. 11 (10): 563–75. doi:10.1038/nrcardio.2014.84. PMID 24958078.