Antithrombin III: Difference between revisions
No edit summary |
m (Protected "Antithrombin III": Bot: Protecting all pages from category Drug ([Edit=Allow only administrators] (indefinite) [Move=Allow only administrators] (indefinite))) |
||
| (30 intermediate revisions by 7 users not shown) | |||
| Line 1: | Line 1: | ||
{{ | {{DrugProjectFormSinglePage | ||
|authorTag={{AL}} | |||
|genericName=Antithrombin III | |||
|aOrAn=a | |||
|drugClass=[[anti-coagulant]] | |||
|indication=[[hereditary antithrombin III deficiency]] | |||
|adverseReactions=[[dizziness]], [[chest discomfort]], [[nausea]], [[dysgeusia]], [[chills]] | |||
|blackBoxWarningTitle=Warning Title | |||
|blackBoxWarningBody=<i><span style="color:#FF0000;">Condition Name:</span></i> (Content) | |||
|fdaLIADAdult======Hereditary Antithrombin III Deficiency===== | |||
* Dosage should be determined on an individual basis based on the pre-therapy plasma ATIII level, in order to increase plasma ATIII levels to the level found in normal human plasma (80-120%). | |||
* Dosage of antithrombin III can be calculated from the following formula: | |||
[[Image:ATIII Dosing Formula.png|450px|left]] | |||
{{clr}} | |||
* The recommendations for dosing are provided as a general guideline for therapy only. The exact loading and maintenance dosages and dosing intervals should be individualized for each subject, based on the individual clinical conditions, response to therapy, and actual plasma ATIII levels achieved. | |||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Antithrombin III in adult patients. | |||
|offLabelAdultNoGuideSupport======Acquired Antithrombin III Deficiency===== | |||
* Severe Sepsis<ref>{{Cite journal | |||
| author = [[B. Eisele]], [[M. Lamy]], [[L. G. Thijs]], [[H. O. Keinecke]], [[H. P. Schuster]], [[F. R. Matthias]], [[F. Fourrier]], [[H. Heinrichs]] & [[U. Delvos]] | |||
| title = Antithrombin III in patients with severe sepsis. A randomized, placebo-controlled, double-blind multicenter trial plus a meta-analysis on all randomized, placebo-controlled, double-blind trials with antithrombin III in severe sepsis | |||
| journal = [[Intensive care medicine]] | |||
| volume = 24 | |||
| issue = 7 | |||
| pages = 663–672 | |||
| year = 1998 | |||
| month = July | |||
| pmid = 9722035 | |||
}}</ref> | |||
:* Loading dose: '''3000 IU''' (in 1 hour) | |||
:* Maintenance dose: '''1500 IU q12h''' for 5 days. | |||
*Shock and DIC | |||
:* Dose was based in the following formula: (100 - antithrombin III activity in %) x body weight (kg). | |||
:* Repeat dose if antithrombin III activity fell below 80%. | |||
== | =====Heparin Resistance===== | ||
* In patients with unstable angina:<ref>{{Cite journal | |||
| author = [[M. Rossi]], [[L. Martinelli]], [[S. Storti]], [[M. Corrado]], [[R. Marra]], [[C. Varano]] & [[R. Schiavello]] | |||
| title = The role of antithrombin III in the perioperative management of the patient with unstable angina | |||
| journal = [[The Annals of thoracic surgery]] | |||
| volume = 68 | |||
| issue = 6 | |||
| pages = 2231–2236 | |||
| year = 1999 | |||
| month = December | |||
| pmid = 10617008 | |||
}}</ref> | |||
:* '''3000 IU + [[heparin]] 300 U/kg''' | |||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Antithrombin III in pediatric patients. | |||
|offLabelPedNoGuideSupport======Disseminated Intravascular Coagulation===== | |||
* Dosing Information | |||
== | :* Children (1 month to 5 years): '''250 units IV q8h''', with or without [[heparin]]. <ref>{{Cite journal | ||
| author = [[T. Hanada]], [[T. Abe]] & [[H. Takita]] | |||
| title = Antithrombin III concentrates for treatment of disseminated intravascular coagulation in children | |||
| journal = [[The American journal of pediatric hematology/oncology]] | |||
| volume = 7 | |||
| issue = 1 | |||
| pages = 3–8 | |||
| year = 1985 | |||
| month = Spring | |||
| pmid = 4037242 | |||
}}</ref> | |||
:* Newborn infants: '''40 units/kg/day + [[heparin]] 200 units/kg/day'''<ref>{{Cite journal | |||
| author = [[R. von Kries]], [[H. Stannigel]] & [[U. Gobel]] | |||
| title = Anticoagulant therapy by continuous [[heparin]]-antithrombin III infusion in newborns with disseminated intravascular coagulation | |||
| journal = [[European journal of pediatrics]] | |||
| volume = 144 | |||
| issue = 2 | |||
| pages = 191–194 | |||
| year = 1985 | |||
| month = July | |||
| pmid = 4043133 | |||
}}</ref> | |||
|contraindications=*Antithrombin III is contraindicated in patients with hypersensitivity to antithrombin III. | |||
|warnings=*Because antithrombin III is made from human [[plasma]], it may carry a risk of transmitting infectious agents, e.g. viruses and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent. | |||
*No cases of transmission of viral diseases or CJD have ever been identified for antithrombin III. | |||
*Inform patients that antithrombin III is made from human [[plasma]] and may contain infectious agents that can cause disease. | |||
*While the risk that antithrombin III can transmit an infectious agent has been reduced by screening [[plasma]] donors for prior exposure, testing donated [[plasma]], and by inactivating or removing pathogens during manufacturing, patients should report any symptoms that concern them. | |||
*All infections thought by a physician possibly to have been transmitted by this product should be reported by the physician or other healthcare provider. | |||
* The [[anticoagulant]] effect of [[heparin]] is enhanced by concurrent treatment with antithrombin III in patients with hereditary ATIII deficiency. Thus, in order to avoid bleeding, reduced dosage of [[heparin]] is recommended during treatment with antithrombin III. | |||
|clinicalTrials=In clinical studies involving antithrombin III, adverse reactions were reported in association with 17 of the 340 infusions during the clinical studies. Included were: | |||
* [[Dizziness]] | |||
*[[Chest discomfort]] | |||
*[[Nausea]] | |||
*[[Dysgeusia]] | |||
*[[Chills]] | |||
*[[Abdominal pain]] | |||
*[[Dyspnea]] | |||
*[[Chest pain]] | |||
*[[Blurred vision]] | |||
*[[Intestinal dilatation]] | |||
*[[Urticaria]] | |||
*[[Pyrexia]] | |||
*Wound secretion and [[hematoma]] | |||
= | If adverse reactions are experienced, the infusion rate should be decreased, or if indicated, the infusion should be interrupted until symptoms abate. | ||
|drugInteractions=*The [[anticoagulant]] effect of [[heparin]] is enhanced by concurrent treatment with antithrombin III in patients with hereditary ATIII deficiency. | |||
*Thus, in order to avoid bleeding, reduced dosage of [[heparin]] is recommended during treatment with antithrombin III. | |||
|FDAPregCat=B | |||
|useInPregnancyFDA=Reproduction studies have been performed in rats and rabbits at doses up to four times the human dose and have revealed no evidence of impaired fertility or harm to the fetus due to antithrombin III. It is not known whether antithrombin III can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed. | |||
|useInPed=Safety and effectiveness in the pediatric population have not been established. The ATIII level in neonates of parents with hereditary ATIII deficiency should be measured immediately after birth. (Fatal neonatal [[thromboembolism]], such as aortic thrombi in children of women with hereditary antithrombin III deficiency, has been reported.) | |||
[[Plasma]] levels of ATIII are lower in neonates than adults, averaging approximately 60% in normal term infants. ATIII levels in premature infants may be much lower. Low [[plasma]] ATIII levels, especially in a premature infant, therefore, do not necessarily indicate hereditary deficiency. It is recommended that testing and treatment with antithrombin III of neonates be discussed with an expert on coagulation. | |||
|administration=*Administer within 3 hours after reconstitution. Do not refrigerate after reconstitution. | |||
*Administer only by the intravenous route. | |||
*Antithrombin III, once reconstituted, should be given alone, without mixing with other agents or diluting solutions. | |||
*Product administration and handling of the needles must be done with caution. [[Percutaneous]] puncture with a needle contaminated with blood can transmit infectious virus including [[HIV]] ([[AIDS]]) and [[hepatitis]]. Obtain immediate medical attention if injury occurs. | |||
=====Reconstitution===== | |||
Vacuum Transfer<br> | |||
Note: Aseptic technique should be carefully followed. All needles and vial tops that will come into contact with the product to be administered via the intravenous route should not come in contact with any nonsterile surface. Any contaminated needles should be discarded by placing in a puncture-proof container and new equipment should be used. | |||
# Antithrombin III and diluent should be at room temperature before reconstitution. | |||
# Remove shrink band from product vial. '''If the shrink band is absent or shows signs of tampering, do not use the product and notify Grifols Therapeutics Inc. immediately.''' | |||
# Remove the plastic flip tops from each vial (Fig. A). Cleanse vial tops (grey stoppers) with alcohol swab and allow surface to dry. After cleaning, do not allow anything to touch the stopper. | |||
# Carefully remove the plastic sheath from the short end of the transfer needle. Insert the exposed needle into the diluent vial to the hub (Fig. B). | |||
# Carefully grip the sheath of the other end of the transfer needle and twist to remove it. | |||
# Invert the diluent vial and insert the attached needle into the antithrombin III vial at a 45° angle (Fig. C). This will direct the stream of diluent against the wall of the vial and minimize foaming. The vacuum will draw the diluent into the antithrombin III vial.* | |||
# When diluent transfer is complete, remove the diluent vial and transfer needle (Fig. D). | |||
# Immediately after adding the diluent, swirl continuously until completely dissolved (Fig. E). Some foaming may occur, but attempt to avoid excessive foaming. The vial should then be visually inspected for particulate matter and discoloration prior to administration. | |||
# Clean the top of the vial of reconstituted antithrombin III again with alcohol swab and let surface dry. | |||
# Attach the filter needle (from the package) to sterile syringe. Withdraw the antithrombin III solution into the syringe through the filter needle (Fig. F). | |||
# Remove the filter needle from the [[syringe]] and replace with an appropriate injection or [[butterfly needle]] for administration. Discard filter needle into a puncture-proof container. | |||
# If the same patient is using more than one vial of antithrombin III, the contents of multiple vials may be drawn into the same syringe through the filter needles provided. | |||
*If vacuum is lost in the antithrombin III vial during reconstitution, use a sterile syringe to remove the sterile water from the diluent vial and inject it into the antithrombin III vial, directing the stream of fluid against the wall of the vial. | |||
[[File:Antithrombin_III_03.png|thumb|600px|left]] | |||
{{clr}} | |||
=====Rate of Administration===== | |||
The rate of administration should be adapted to the response of the individual patient, but administration of the entire dose in 10 to 20 minutes is generally well tolerated. | |||
|monitoring=*It is recommended that following an initial dose of Antithrombin III, plasma levels of ATIII be initially monitored at least every 12 hours and before the next infusion of Antithrombin IIII to maintain plasma ATIII levels greater than 80%. | |||
*Measure preinfusion and 20 minutes postinfusion (peak) [[plasma]] ATIII levels following the initial loading dose, [[plasma]] ATIII level after 12 hours, then preceding the next infusion (trough level). | |||
*Subsequently measure ATIII levels preceding and 20 minutes after each infusion until predictable peak and trough levels have been achieved, generally between 80%–120%. | |||
* [[Plasma]] levels between 80%–120% may be maintained by administration of maintenance doses of 60% of the initial loading dose, administered every 24 hours. | |||
* Adjustments in the maintenance dose and/or interval between doses should be made based on actual [[plasma]] ATIII levels achieved. | |||
*In some situations, e.g., following surgery, hemorrhage or acute thrombosis, and during intravenous [[heparin]] administration, the half-life of antithrombin III (Human) has been reported to be shortened. In such conditions, [[plasma]] ATIII levels should be monitored more frequently, and antithrombin III administered as necessary. | |||
|overdose=There is limited information regarding overdose of antithrombin III in the drug label. | |||
|mechAction=Antithrombin III, an alpha2-glycoprotein of molecular weight 58,000, is normally present in human [[plasma]] at a concentration of approximately 12.5 mg/dL and is the major [[plasma]] inhibitor of [[thrombin]]. | |||
|structure=There is limited information regarding the structure of antithrombin III in the drug label. | |||
|PD=Inactivation of [[thrombin]] by ATIII occurs by formation of a covalent bond resulting in an inactive 1:1 stoichiometric complex between the two, involving an interaction of the active [[serine]] of [[thrombin]] and an [[arginine]] reactive site on ATIII. ATIII is also capable of inactivating other components of the coagulation cascade including factors IXa, Xa, XIa, and XIIa, as well as plasmin. | |||
|PK=There is limited information regarding pharmacokinetics of antithrombin III in the drug label. | |||
|nonClinToxic=There is limited information regarding nonclinical toxicology of antithrombin III in the drug label. | |||
|clinicalStudies=In clinical studies of antithrombin III conducted in 10 asymptomatic subjects with hereditary deficiency of ATIII, the mean in vivo recovery of ATIII was 1.6% per unit per kg administered based on immunologic ATIII assays, and 1.4% per unit per kg administered based on functional ATIII assays. The mean 50% disappearance time (the time to fall to 50% of the peak plasma level following an initial administration) was approximately 22 hours and the biologic half-life was 2.5 days based on immunologic assays and 3.8 days based on functional assays of ATIII. These values are similar to the half-life for radiolabeled Antithrombin III (Human) reported in the literature of 2.8–4.8 days. | |||
In clinical studies of antithrombin III, none of the 13 patients with hereditary ATIII deficiency and histories of [[thromboembolism]] treated prophylactically on 16 separate occasions with antithrombin III for high [[thrombotic]] risk situations (11 surgical procedures, 5 deliveries) developed a thrombotic complication. [[Heparin]] was also administered in 3 of the 11 surgical procedures. Eight patients with hereditary ATIII deficiency were treated therapeutically with antithrombin III as well as [[heparin]] for major thrombotic or [[thromboembolic]] complications, with seven patients recovering. Treatment with antithrombin III reversed [[heparin]] resistance in two patients with hereditary ATIII deficiency being treated for [[thrombosis]] or [[thromboembolism]]. | |||
During clinical investigation of antithrombin III, none of 12 subjects monitored for a median of 8 months (range 2–19 months) after receiving antithrombin III became antibody positive to [[human immunodeficiency virus]] ([[HIV]]-1). None of 14 subjects monitored for ≥ 3 months demonstrated any evidence of [[hepatitis]], either non-A, non-B [[hepatitis]] or [[hepatitis B]]. | |||
|howSupplied=Antithrombin III is supplied in a kit containing one single use vial of Antithrombin III lyophilized powder, one vial of Sterile Water for Injection, USP, one sterile double-ended transfer needle, and one sterile filter needle. The total activity of ATIII in International Units is stated on the label of the Antithrombin III vial. | |||
[[ | [[image:Antithrombin III 04.png|500px|left]] | ||
{{clr}} | |||
|storage=Antithrombin III should be stored at temperatures not to exceed 25°C (77°F). Freezing should be avoided as breakage of the diluent bottle might occur. | |||
|alcohol=Alcohol-Antithrombin III interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |||
|brandNames= | |||
Thrombate III | |||
|nlmPatientInfo=(Link to patient information page) | |||
|drugShortage=Drug Shortage | |||
}} | |||
{{LabelImage | |||
|fileName=Antithrombin III label.jpg | |||
}} | |||
[[Category:Drug]] | |||
[[Category:Cardiovascular Drugs]] | |||
[[Category:Anticoagulants]] | |||
Latest revision as of 17:40, 18 August 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alejandro Lemor, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Antithrombin III is a anti-coagulant that is FDA approved for the {{{indicationType}}} of hereditary antithrombin III deficiency. Common adverse reactions include dizziness, chest discomfort, nausea, dysgeusia, chills.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Hereditary Antithrombin III Deficiency
- Dosage should be determined on an individual basis based on the pre-therapy plasma ATIII level, in order to increase plasma ATIII levels to the level found in normal human plasma (80-120%).
- Dosage of antithrombin III can be calculated from the following formula:
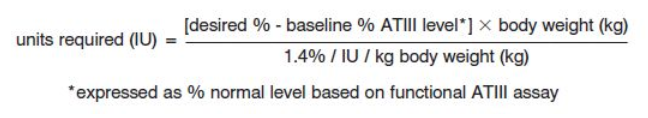
- The recommendations for dosing are provided as a general guideline for therapy only. The exact loading and maintenance dosages and dosing intervals should be individualized for each subject, based on the individual clinical conditions, response to therapy, and actual plasma ATIII levels achieved.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Antithrombin III in adult patients.
Non–Guideline-Supported Use
Acquired Antithrombin III Deficiency
- Severe Sepsis[1]
- Loading dose: 3000 IU (in 1 hour)
- Maintenance dose: 1500 IU q12h for 5 days.
- Shock and DIC
- Dose was based in the following formula: (100 - antithrombin III activity in %) x body weight (kg).
- Repeat dose if antithrombin III activity fell below 80%.
Heparin Resistance
- In patients with unstable angina:[2]
- 3000 IU + heparin 300 U/kg
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Antithrombin III FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Antithrombin III in pediatric patients.
Non–Guideline-Supported Use
Disseminated Intravascular Coagulation
- Dosing Information
Contraindications
- Antithrombin III is contraindicated in patients with hypersensitivity to antithrombin III.
Warnings
- Because antithrombin III is made from human plasma, it may carry a risk of transmitting infectious agents, e.g. viruses and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent.
- No cases of transmission of viral diseases or CJD have ever been identified for antithrombin III.
- Inform patients that antithrombin III is made from human plasma and may contain infectious agents that can cause disease.
- While the risk that antithrombin III can transmit an infectious agent has been reduced by screening plasma donors for prior exposure, testing donated plasma, and by inactivating or removing pathogens during manufacturing, patients should report any symptoms that concern them.
- All infections thought by a physician possibly to have been transmitted by this product should be reported by the physician or other healthcare provider.
- The anticoagulant effect of heparin is enhanced by concurrent treatment with antithrombin III in patients with hereditary ATIII deficiency. Thus, in order to avoid bleeding, reduced dosage of heparin is recommended during treatment with antithrombin III.
Adverse Reactions
Clinical Trials Experience
In clinical studies involving antithrombin III, adverse reactions were reported in association with 17 of the 340 infusions during the clinical studies. Included were:
- Dizziness
- Chest discomfort
- Nausea
- Dysgeusia
- Chills
- Abdominal pain
- Dyspnea
- Chest pain
- Blurred vision
- Intestinal dilatation
- Urticaria
- Pyrexia
- Wound secretion and hematoma
If adverse reactions are experienced, the infusion rate should be decreased, or if indicated, the infusion should be interrupted until symptoms abate.
Postmarketing Experience
There is limited information regarding Antithrombin III Postmarketing Experience in the drug label.
Drug Interactions
- The anticoagulant effect of heparin is enhanced by concurrent treatment with antithrombin III in patients with hereditary ATIII deficiency.
- Thus, in order to avoid bleeding, reduced dosage of heparin is recommended during treatment with antithrombin III.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): B
Reproduction studies have been performed in rats and rabbits at doses up to four times the human dose and have revealed no evidence of impaired fertility or harm to the fetus due to antithrombin III. It is not known whether antithrombin III can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Antithrombin III in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Antithrombin III during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Antithrombin III in women who are nursing.
Pediatric Use
Safety and effectiveness in the pediatric population have not been established. The ATIII level in neonates of parents with hereditary ATIII deficiency should be measured immediately after birth. (Fatal neonatal thromboembolism, such as aortic thrombi in children of women with hereditary antithrombin III deficiency, has been reported.)
Plasma levels of ATIII are lower in neonates than adults, averaging approximately 60% in normal term infants. ATIII levels in premature infants may be much lower. Low plasma ATIII levels, especially in a premature infant, therefore, do not necessarily indicate hereditary deficiency. It is recommended that testing and treatment with antithrombin III of neonates be discussed with an expert on coagulation.
Geriatic Use
There is no FDA guidance on the use of Antithrombin III in geriatric settings.
Gender
There is no FDA guidance on the use of Antithrombin III with respect to specific gender populations.
Race
There is no FDA guidance on the use of Antithrombin III with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Antithrombin III in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Antithrombin III in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Antithrombin III in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Antithrombin III in patients who are immunocompromised.
Administration and Monitoring
Administration
- Administer within 3 hours after reconstitution. Do not refrigerate after reconstitution.
- Administer only by the intravenous route.
- Antithrombin III, once reconstituted, should be given alone, without mixing with other agents or diluting solutions.
- Product administration and handling of the needles must be done with caution. Percutaneous puncture with a needle contaminated with blood can transmit infectious virus including HIV (AIDS) and hepatitis. Obtain immediate medical attention if injury occurs.
Reconstitution
Vacuum Transfer
Note: Aseptic technique should be carefully followed. All needles and vial tops that will come into contact with the product to be administered via the intravenous route should not come in contact with any nonsterile surface. Any contaminated needles should be discarded by placing in a puncture-proof container and new equipment should be used.
- Antithrombin III and diluent should be at room temperature before reconstitution.
- Remove shrink band from product vial. If the shrink band is absent or shows signs of tampering, do not use the product and notify Grifols Therapeutics Inc. immediately.
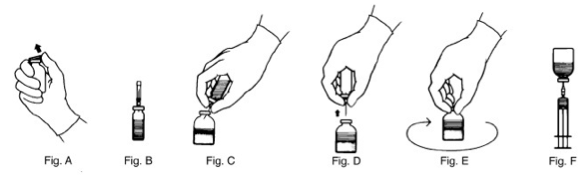
- Remove the plastic flip tops from each vial (Fig. A). Cleanse vial tops (grey stoppers) with alcohol swab and allow surface to dry. After cleaning, do not allow anything to touch the stopper.
- Carefully remove the plastic sheath from the short end of the transfer needle. Insert the exposed needle into the diluent vial to the hub (Fig. B).
- Carefully grip the sheath of the other end of the transfer needle and twist to remove it.
- Invert the diluent vial and insert the attached needle into the antithrombin III vial at a 45° angle (Fig. C). This will direct the stream of diluent against the wall of the vial and minimize foaming. The vacuum will draw the diluent into the antithrombin III vial.*
- When diluent transfer is complete, remove the diluent vial and transfer needle (Fig. D).
- Immediately after adding the diluent, swirl continuously until completely dissolved (Fig. E). Some foaming may occur, but attempt to avoid excessive foaming. The vial should then be visually inspected for particulate matter and discoloration prior to administration.
- Clean the top of the vial of reconstituted antithrombin III again with alcohol swab and let surface dry.
- Attach the filter needle (from the package) to sterile syringe. Withdraw the antithrombin III solution into the syringe through the filter needle (Fig. F).
- Remove the filter needle from the syringe and replace with an appropriate injection or butterfly needle for administration. Discard filter needle into a puncture-proof container.
- If the same patient is using more than one vial of antithrombin III, the contents of multiple vials may be drawn into the same syringe through the filter needles provided.
- If vacuum is lost in the antithrombin III vial during reconstitution, use a sterile syringe to remove the sterile water from the diluent vial and inject it into the antithrombin III vial, directing the stream of fluid against the wall of the vial.
Rate of Administration
The rate of administration should be adapted to the response of the individual patient, but administration of the entire dose in 10 to 20 minutes is generally well tolerated.
Monitoring
- It is recommended that following an initial dose of Antithrombin III, plasma levels of ATIII be initially monitored at least every 12 hours and before the next infusion of Antithrombin IIII to maintain plasma ATIII levels greater than 80%.
- Measure preinfusion and 20 minutes postinfusion (peak) plasma ATIII levels following the initial loading dose, plasma ATIII level after 12 hours, then preceding the next infusion (trough level).
- Subsequently measure ATIII levels preceding and 20 minutes after each infusion until predictable peak and trough levels have been achieved, generally between 80%–120%.
- Plasma levels between 80%–120% may be maintained by administration of maintenance doses of 60% of the initial loading dose, administered every 24 hours.
- Adjustments in the maintenance dose and/or interval between doses should be made based on actual plasma ATIII levels achieved.
- In some situations, e.g., following surgery, hemorrhage or acute thrombosis, and during intravenous heparin administration, the half-life of antithrombin III (Human) has been reported to be shortened. In such conditions, plasma ATIII levels should be monitored more frequently, and antithrombin III administered as necessary.
IV Compatibility
There is limited information regarding the compatibility of Antithrombin III and IV administrations.
Overdosage
There is limited information regarding overdose of antithrombin III in the drug label.
Pharmacology
There is limited information regarding Antithrombin III Pharmacology in the drug label.
Mechanism of Action
Antithrombin III, an alpha2-glycoprotein of molecular weight 58,000, is normally present in human plasma at a concentration of approximately 12.5 mg/dL and is the major plasma inhibitor of thrombin.
Structure
There is limited information regarding the structure of antithrombin III in the drug label.
Pharmacodynamics
Inactivation of thrombin by ATIII occurs by formation of a covalent bond resulting in an inactive 1:1 stoichiometric complex between the two, involving an interaction of the active serine of thrombin and an arginine reactive site on ATIII. ATIII is also capable of inactivating other components of the coagulation cascade including factors IXa, Xa, XIa, and XIIa, as well as plasmin.
Pharmacokinetics
There is limited information regarding pharmacokinetics of antithrombin III in the drug label.
Nonclinical Toxicology
There is limited information regarding nonclinical toxicology of antithrombin III in the drug label.
Clinical Studies
In clinical studies of antithrombin III conducted in 10 asymptomatic subjects with hereditary deficiency of ATIII, the mean in vivo recovery of ATIII was 1.6% per unit per kg administered based on immunologic ATIII assays, and 1.4% per unit per kg administered based on functional ATIII assays. The mean 50% disappearance time (the time to fall to 50% of the peak plasma level following an initial administration) was approximately 22 hours and the biologic half-life was 2.5 days based on immunologic assays and 3.8 days based on functional assays of ATIII. These values are similar to the half-life for radiolabeled Antithrombin III (Human) reported in the literature of 2.8–4.8 days.
In clinical studies of antithrombin III, none of the 13 patients with hereditary ATIII deficiency and histories of thromboembolism treated prophylactically on 16 separate occasions with antithrombin III for high thrombotic risk situations (11 surgical procedures, 5 deliveries) developed a thrombotic complication. Heparin was also administered in 3 of the 11 surgical procedures. Eight patients with hereditary ATIII deficiency were treated therapeutically with antithrombin III as well as heparin for major thrombotic or thromboembolic complications, with seven patients recovering. Treatment with antithrombin III reversed heparin resistance in two patients with hereditary ATIII deficiency being treated for thrombosis or thromboembolism.
During clinical investigation of antithrombin III, none of 12 subjects monitored for a median of 8 months (range 2–19 months) after receiving antithrombin III became antibody positive to human immunodeficiency virus (HIV-1). None of 14 subjects monitored for ≥ 3 months demonstrated any evidence of hepatitis, either non-A, non-B hepatitis or hepatitis B.
How Supplied
Antithrombin III is supplied in a kit containing one single use vial of Antithrombin III lyophilized powder, one vial of Sterile Water for Injection, USP, one sterile double-ended transfer needle, and one sterile filter needle. The total activity of ATIII in International Units is stated on the label of the Antithrombin III vial.

Storage
Antithrombin III should be stored at temperatures not to exceed 25°C (77°F). Freezing should be avoided as breakage of the diluent bottle might occur.
Images
Drug Images
{{#ask: Page Name::Antithrombin III |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Antithrombin III |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Antithrombin III Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Antithrombin III interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Thrombate III
Look-Alike Drug Names
There is limited information regarding Antithrombin III Look-Alike Drug Names in the drug label.
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ B. Eisele, M. Lamy, L. G. Thijs, H. O. Keinecke, H. P. Schuster, F. R. Matthias, F. Fourrier, H. Heinrichs & U. Delvos (1998). "Antithrombin III in patients with severe sepsis. A randomized, placebo-controlled, double-blind multicenter trial plus a meta-analysis on all randomized, placebo-controlled, double-blind trials with antithrombin III in severe sepsis". Intensive care medicine. 24 (7): 663–672. PMID 9722035. Unknown parameter
|month=ignored (help) - ↑ M. Rossi, L. Martinelli, S. Storti, M. Corrado, R. Marra, C. Varano & R. Schiavello (1999). "The role of antithrombin III in the perioperative management of the patient with unstable angina". The Annals of thoracic surgery. 68 (6): 2231–2236. PMID 10617008. Unknown parameter
|month=ignored (help) - ↑ T. Hanada, T. Abe & H. Takita (1985). "Antithrombin III concentrates for treatment of disseminated intravascular coagulation in children". The American journal of pediatric hematology/oncology. 7 (1): 3–8. PMID 4037242. Unknown parameter
|month=ignored (help) - ↑ R. von Kries, H. Stannigel & U. Gobel (1985). "Anticoagulant therapy by continuous heparin-antithrombin III infusion in newborns with disseminated intravascular coagulation". European journal of pediatrics. 144 (2): 191–194. PMID 4043133. Unknown parameter
|month=ignored (help)
{{#subobject:
|Label Page=Antithrombin III |Label Name=Antithrombin III label.jpg
}}