Androgen insensitivity syndrome pathophysiology: Difference between revisions
| (113 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
__NOTOC__ | __NOTOC__ | ||
{{Androgen insensitivity syndrome}} | {{Androgen insensitivity syndrome}} | ||
{{CMG}}; {{AE}} | {{CMG}}; {{AE}} {{ARK}} | ||
==Overview== | ==Overview== | ||
Androgen insensitivity syndrome is due to [[hormone]] resistance which may be due to defective [[androgen receptor]] ([[Androgen receptor|AR]]) function by either abnormal [[androgen receptor]] ([[Androgen receptor|AR]]) binding, decreased receptor binding, or impaired [[androgen receptor]] ([[Androgen receptor|AR]]) binding. AIS is an [[X linked]] disorder. The development of androgen insensitivity syndrome is a result of [[genetic mutations]] of the [[androgen receptor]] ([[Androgen receptor|AR]]) gene located on the chromosome Xq11-12. Associated conditions include [[primary amenorrhea]], [[infertility]] and [[dyspareunia]]. | |||
==Pathophysiology== | ==Pathophysiology== | ||
Androgen | ===Pathogenesis=== | ||
*Androgen resistance may develop during fetal development and after birth. | |||
*The hormone resistance may be due to defective [[androgen receptor]] ([[Androgen receptor|AR]]) function by either abnormal [[androgen receptor]] ([[Androgen receptor|AR]]) binding, decreased receptor binding, or impaired [[androgen receptor]] ([[Androgen receptor|AR]]) binding. <ref name="FlierUnderhill1992">{{cite journal|last1=Flier|first1=Jeffrey S.|last2=Underhill|first2=Lisa H.|last3=Griffin|first3=James E.|title=Androgen Resistance — The Clinical and Molecular Spectrum|journal=New England Journal of Medicine|volume=326|issue=9|year=1992|pages=611–618|issn=0028-4793|doi=10.1056/NEJM199202273260906}}</ref><ref name="BrownMaes1982">{{cite journal|last1=Brown|first1=Terry R.|last2=Maes|first2=Marc|last3=Rothwell|first3=Stephen W.|last4=Migeon|first4=Claude J.|title=Human Complete Androgen Insensitivity with Normal Dihydrotestosterone Receptor Binding Capacity in Cultured Genital Skin Fibroblasts: Evidence for a Qualitative Abnormality of the Receptor*|journal=The Journal of Clinical Endocrinology & Metabolism|volume=55|issue=1|year=1982|pages=61–69|issn=0021-972X|doi=10.1210/jcem-55-1-61}}</ref><ref name="Griffin1979">{{cite journal|last1=Griffin|first1=James E.|title=Testicular Feminization Associated with a Thermolabile Androgen Receptor in Cultured Human Fibroblasts|journal=Journal of Clinical Investigation|volume=64|issue=6|year=1979|pages=1624–1631|issn=0021-9738|doi=10.1172/JCI109624}}</ref> | |||
*A spectrum of [[phenotypes]] may be caused due to [[missense mutations]] in the [[androgen receptor]] ([[Androgen receptor|AR]]) [[protein]]. The two important domains of the [[receptor protein]] namely DBD and LDB domains are the ones wherein the most frequent [[missense mutations]] are found.<ref name="pmid24339553">{{cite journal |vauthors=Kota SK, Gayatri K, Kota SK, Jammula S |title=Genetic analysis of a family with complete androgen insensitivity syndrome |journal=Indian J Hum Genet |volume=19 |issue=3 |pages=355–7 |year=2013 |pmid=24339553 |pmc=3841565 |doi=10.4103/0971-6866.120820 |url=}}</ref><ref name="pmid2626022">{{cite journal |vauthors=Brinkmann AO, Faber PW, van Rooij HC, Kuiper GG, Ris C, Klaassen P, van der Korput JA, Voorhorst MM, van Laar JH, Mulder E |title=The human androgen receptor: domain structure, genomic organization and regulation of expression |journal=J. Steroid Biochem. |volume=34 |issue=1-6 |pages=307–10 |year=1989 |pmid=2626022 |doi= |url=}}</ref> | |||
*The [[phenotype|phenotypic]] variability impacts and translates the degree to which ligand-binding and [[receptor]] functions are disrupted by different substitutions.<ref name="pmid24339553">{{cite journal |vauthors=Kota SK, Gayatri K, Kota SK, Jammula S |title=Genetic analysis of a family with complete androgen insensitivity syndrome |journal=Indian J Hum Genet |volume=19 |issue=3 |pages=355–7 |year=2013 |pmid=24339553 |pmc=3841565 |doi=10.4103/0971-6866.120820 |url=}}</ref><ref name="pmid1752359">{{cite journal |vauthors=McPhaul MJ, Marcelli M, Tilley WD, Griffin JE, Wilson JD |title=Androgen resistance caused by mutations in the androgen receptor gene |journal=FASEB J. |volume=5 |issue=14 |pages=2910–5 |year=1991 |pmid=1752359 |doi= |url=}}</ref> | |||
*The genetic background has an influence on the resulting [[phenotype]] as a result of the same [[mutation]] which may lead to different forms of AIS within a family.<ref name="pmid24339553">{{cite journal |vauthors=Kota SK, Gayatri K, Kota SK, Jammula S |title=Genetic analysis of a family with complete androgen insensitivity syndrome |journal=Indian J Hum Genet |volume=19 |issue=3 |pages=355–7 |year=2013 |pmid=24339553 |pmc=3841565 |doi=10.4103/0971-6866.120820 |url=}}</ref><ref name="pmid9245853">{{cite journal |vauthors=Evans BA, Hughes IA, Bevan CL, Patterson MN, Gregory JW |title=Phenotypic diversity in siblings with partial androgen insensitivity syndrome |journal=Arch. Dis. Child. |volume=76 |issue=6 |pages=529–31 |year=1997 |pmid=9245853 |pmc=1717223 |doi= |url=}}</ref> | |||
*Mutations in the [[androgen receptor]] ([[Androgen receptor|AR]]) gene helps as a trusted tool for the diagnosis and molecular subclassification of AIS. The resulting [[phenotype]] is affected by the kind of amino acid substitution occurring due to [[mutation]].<ref name="pmid24339553">{{cite journal |vauthors=Kota SK, Gayatri K, Kota SK, Jammula S |title=Genetic analysis of a family with complete androgen insensitivity syndrome |journal=Indian J Hum Genet |volume=19 |issue=3 |pages=355–7 |year=2013 |pmid=24339553 |pmc=3841565 |doi=10.4103/0971-6866.120820 |url=}}</ref> | |||
*Information regarding the mutation in the [[androgen receptor]] ([[Androgen receptor|AR]]) and its functional consequences helps in determining the genotype-phenotype correlation, to improve and better manage the cases of male [[pseudohermaphroditism]] pertaining to surgery of the [[genitalia]], gonadectomy and gender assignment. <ref name="pmid24339553">{{cite journal |vauthors=Kota SK, Gayatri K, Kota SK, Jammula S |title=Genetic analysis of a family with complete androgen insensitivity syndrome |journal=Indian J Hum Genet |volume=19 |issue=3 |pages=355–7 |year=2013 |pmid=24339553 |pmc=3841565 |doi=10.4103/0971-6866.120820 |url=}}</ref> | |||
*The lack of virilization of external genitalia is due to the failure of genital folds to fuse and to form [[scrotum]] and [[penis]]. | |||
*The agenesis of the [[fallopian tubes]], [[uterus]], [[cervix]] and proximal [[vagina]] is due to the simultaneous [[testicular]] production of [[mullerian inhibiting factor]] which regresses the mullerian structures. | |||
*In CAIS (complete androgen insensitivity syndrome), the development of the [[wolffian duct]] and the differentiation of male external [[genitalia]] do not occur correctly, and the [[Mullerian ducts]] regress due to the presence of anti-Mullerian hormone (AMH) produced by the [[sertoli cells]] of normally developed [[gonads]]. The residual [[Mullerian structures]] exist in approximately one third of patients. <ref name="pmid18930210">{{cite journal |vauthors=Nichols JL, Bieber EJ, Gell JS |title=Case of sisters with complete androgen insensitivity syndrome and discordant Müllerian remnants |journal=Fertil. Steril. |volume=91 |issue=3 |pages=932.e15–8 |year=2009 |pmid=18930210 |doi=10.1016/j.fertnstert.2008.09.027 |url=}}</ref> <ref name="pmid25191030">{{cite journal |vauthors=Ozdemir O, Sari ME, Akmut E, Selimova V, Unal T, Atalay CR |title=Complete androgen insensitivity syndrome with a large gonadal serous papillary cystadenofibroma |journal=J Hum Reprod Sci |volume=7 |issue=2 |pages=148–50 |year=2014 |pmid=25191030 |pmc=4150143 |doi=10.4103/0974-1208.138875 |url=}}</ref> | |||
*The female [[phenotype]] along with [[breast]] development is a result of the peripheral [[aromatization]] of [[testosterone]] into [[estrogen]].<ref name="pmid17161333">{{cite journal |vauthors=Hughes IA, Deeb A |title=Androgen resistance |journal=Best Pract. Res. Clin. Endocrinol. Metab. |volume=20 |issue=4 |pages=577–98 |year=2006 |pmid=17161333 |doi=10.1016/j.beem.2006.11.003 |url=}}</ref> | |||
*As a result of the defective [[androgen receptor]] ([[Androgen receptor|AR]]) due to imperfect feedback mechanism of [[testosterone]] at the [[pituitary]] and [[hypothalamus]] there are elevated levels of serum [[testosterone]], [[FSH]] and [[LH]] observed. | |||
*[[Testosterone]] biosynthetic defects are ruled out by the normal serum 17α hydroxyprogesterone, [[DHEA]] and [[androstenedione]] levels.<ref name="pmid9389232">{{cite journal |vauthors=Viner RM, Teoh Y, Williams DM, Patterson MN, Hughes IA |title=Androgen insensitivity syndrome: a survey of diagnostic procedures and management in the UK |journal=Arch. Dis. Child. |volume=77 |issue=4 |pages=305–9 |year=1997 |pmid=9389232 |pmc=1717340 |doi= |url=}}</ref> | |||
*Sufficient peripheral conversion of [[testosterone]] is indicative of normal [[estradiol]] level. | |||
[[Image:Androgen_dependencies_of_male_genital_tissues.png|thumb|600px|left|By Jonathan.Marcus (Own work) [CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0) or GFDL (http://www.gnu.org/copyleft/fdl.html)], via Wikimedia Commons]] | |||
[[Image:Human_sexual_differentiation.gif|thumb|600px|center|By Jonathan.Marcus (Own work) [CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0) or GFDL (http://www.gnu.org/copyleft/fdl.html)], via Wikimedia Commons]] | |||
===Genetics=== | ===Genetics=== | ||
*AIS is an [[X linked]] disorder. <ref name="pmid28670533">{{cite journal |vauthors=Akella RR |title=Mutational Analysis of Androgen Receptor Gene in Two Families with Androgen Insensitivity |journal=Indian J Endocrinol Metab |volume=21 |issue=4 |pages=520–523 |year=2017 |pmid=28670533 |pmc=5477437 |doi=10.4103/ijem.IJEM_345_16 |url=}}</ref> | |||
*A high proportion of [[De novo mutation|De novo mutations]] arise after the [[zygote]] stage which occur at a high rate within the [[androgen receptor]] ([[Androgen receptor|AR]]) gene.<ref name="pmid28670533">{{cite journal |vauthors=Akella RR |title=Mutational Analysis of Androgen Receptor Gene in Two Families with Androgen Insensitivity |journal=Indian J Endocrinol Metab |volume=21 |issue=4 |pages=520–523 |year=2017 |pmid=28670533 |pmc=5477437 |doi=10.4103/ijem.IJEM_345_16 |url=}}</ref> | |||
*Spontaneous [[mutations]] in the [[androgen receptor]] ([[Androgen receptor|AR]]) gene results in androgen insensitivity syndrome even without any family history.<ref name="pmid28670533">{{cite journal |vauthors=Akella RR |title=Mutational Analysis of Androgen Receptor Gene in Two Families with Androgen Insensitivity |journal=Indian J Endocrinol Metab |volume=21 |issue=4 |pages=520–523 |year=2017 |pmid=28670533 |pmc=5477437 |doi=10.4103/ijem.IJEM_345_16 |url=}}</ref> | |||
*The development of androgen insensitivity syndrome is a result of [[genetic mutations]] of the [[androgen receptor]] ([[Androgen receptor|AR]]) gene located on the chromosome Xq11-12.<ref name="pmid24339553">{{cite journal |vauthors=Kota SK, Gayatri K, Kota SK, Jammula S |title=Genetic analysis of a family with complete androgen insensitivity syndrome |journal=Indian J Hum Genet |volume=19 |issue=3 |pages=355–7 |year=2013 |pmid=24339553 |pmc=3841565 |doi=10.4103/0971-6866.120820 |url=}}</ref><ref name="pmid28670533">{{cite journal |vauthors=Akella RR |title=Mutational Analysis of Androgen Receptor Gene in Two Families with Androgen Insensitivity |journal=Indian J Endocrinol Metab |volume=21 |issue=4 |pages=520–523 |year=2017 |pmid=28670533 |pmc=5477437 |doi=10.4103/ijem.IJEM_345_16 |url=}}</ref> | |||
*Different [[mutations]] in the [[androgen receptor]] ([[Androgen receptor|AR]]) gene leads to varied clinical [[phenotypes]].<ref name="pmid26806084">{{cite journal |vauthors=Li L, Liu WM, Liu MX, Zheng SQ, Zhang JX, Che FY, Liu SG |title=A missense mutation in the androgen receptor gene causing androgen insensitivity syndrome in a Chinese family |journal=Asian J. Androl. |volume=19 |issue=2 |pages=260–261 |year=2017 |pmid=26806084 |pmc=5312231 |doi=10.4103/1008-682X.172647 |url=}}</ref><ref name="Brinkmann2001">{{cite journal|last1=Brinkmann|first1=Albert O.|title=Molecular basis of androgen insensitivity|journal=Molecular and Cellular Endocrinology|volume=179|issue=1-2|year=2001|pages=105–109|issn=03037207|doi=10.1016/S0303-7207(01)00466-X}}</ref> | |||
*There have been more than 800 [[mutations]] in the [[androgen receptor]] [[Androgen receptor|(]][[Androgen receptor|AR]][[Androgen receptor|)]] gene reported in AIS patients. <ref name="pmid23044881">{{cite journal| author=Hughes IA, Werner R, Bunch T, Hiort O| title=Androgen insensitivity syndrome. | journal=Semin Reprod Med | year= 2012 | volume= 30 | issue= 5 | pages= 432-42 | pmid=23044881 | doi=10.1055/s-0032-1324728 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=23044881 }} </ref> | |||
[[Image:XlinkRecessiveAIS.png|thumb|600px|center|By U.S. National Library of Medicine; David-Sarah Hopwood (Originally from http://ghr.nlm.nih.gov/) [Public domain], via Wikimedia Commons]] | |||
| | |||
==Associated Conditions== | |||
*[[Primary amenorrhea]] | |||
*[[Infertility]] | |||
*[[Dyspareunia]] | |||
*Common [[Testicular tumor|testicular tumors]] seen in the Androgen insensitivity syndrome are: <ref name="pmid25395750">{{cite journal |vauthors=Bhaskararao G, Himabindu Y, Nayak SR, Sriharibabu M |title=Laparoscopic gonedectomy in a case of complete androgen insensitivity syndrome |journal=J Hum Reprod Sci |volume=7 |issue=3 |pages=221–3 |year=2014 |pmid=25395750 |pmc=4229800 |doi=10.4103/0974-1208.142498 |url=}}</ref> | |||
:*[[Germ cell tumors]] | |||
:*Sex cord tumors ([[seminoma]]) | |||
:*[[Sertoli Cell Tumor|Sertoli cell tumors]] | |||
:*[[Leydig cell tumor|Leydig cell tumors]] | |||
:*[[Hamartomas]] | |||
[[ | ==Gross Pathology== | ||
*Complete androgen insensitivity syndrome in a 30 years old woman presenting with [[primary amenorrhea]].<ref name="pmid28270903">{{cite journal |vauthors=Souhail R, Amine S, Nadia A, Tarik K, Khalid EK, Abdellatif K, Ahmed A |title=Complete androgen insensitivity syndrome or testicular feminization: review of literature based on a case report |journal=Pan Afr Med J |volume=25 |issue= |pages=199 |year=2016 |pmid=28270903 |pmc=5326263 |doi=10.11604/pamj.2016.25.199.10758 |url=}}</ref>. <ref name="pmid26301004">{{cite journal |vauthors=Lachiri B, Hakimi I, Boudhas A, Guelzim K, Kouach J, Oukabli M, Rahali DM, Dehayni M |title=[Complete androgen insensitivity syndrome: report of two cases and review of literature] |language=French |journal=Pan Afr Med J |volume=20 |issue= |pages=400 |year=2015 |pmid=26301004 |pmc=4524922 |doi=10.11604/pamj.2015.20.400.6760 |url=}}</ref> | |||
<gallery> | |||
< | |||
=== | Image: AIS_-_Front and side view of the patient.jpg|Front and side view of the patient by Regragui Souhail et al from The Pan African Medical Journal - ISSN 1937-8688. Pan Afr Med J. 2016;25:199. Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0). <ref name="pmid28270903">{{cite journal| author=Souhail R, Amine S, Nadia A, Tarik K, Khalid EK, Abdellatif K et al.| title=Complete androgen insensitivity syndrome or testicular feminization: review of literature based on a case report. | journal=Pan Afr Med J | year= 2016 | volume= 25 | issue= | pages= 199 | pmid=28270903 | doi=10.11604/pamj.2016.25.199.10758 | pmc=5326263 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=28270903 }} </ref> | ||
=== | Image: AIS_-_Clinical aspect of the vagina.jpg|Clinical aspect of the vagina by Regragui Souhail et al from The Pan African Medical Journal - ISSN 1937-8688. Pan Afr Med J. 2016;25:199. Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0). <ref name="pmid28270903">{{cite journal| author=Souhail R, Amine S, Nadia A, Tarik K, Khalid EK, Abdellatif K et al.| title=Complete androgen insensitivity syndrome or testicular feminization: review of literature based on a case report. | journal=Pan Afr Med J | year= 2016 | volume= 25 | issue= | pages= 199 | pmid=28270903 | doi=10.11604/pamj.2016.25.199.10758 | pmc=5326263 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=28270903 }} </ref> | ||
Image: AIS_-_Intra- abdominal testes - laparoscopic aspect.jpg|Intra- abdominal testes - Laparoscopic aspect by Regragui Souhail et al from The Pan African Medical Journal - ISSN 1937-8688. Pan Afr Med J. 2016;25:199. Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0). <ref name="pmid28270903">{{cite journal| author=Souhail R, Amine S, Nadia A, Tarik K, Khalid EK, Abdellatif K et al.| title=Complete androgen insensitivity syndrome or testicular feminization: review of literature based on a case report. | journal=Pan Afr Med J | year= 2016 | volume= 25 | issue= | pages= 199 | pmid=28270903 | doi=10.11604/pamj.2016.25.199.10758 | pmc=5326263 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=28270903 }} </ref> | |||
Image: AIS_-_The excised testis - Macroscopic aspect.jpg|The excised testis - Macroscopic aspect by Regragui Souhail et al from The Pan African Medical Journal - ISSN 1937-8688. Pan Afr Med J. 2016;25:199. Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0). <ref name="pmid28270903">{{cite journal| author=Souhail R, Amine S, Nadia A, Tarik K, Khalid EK, Abdellatif K et al.| title=Complete androgen insensitivity syndrome or testicular feminization: review of literature based on a case report. | journal=Pan Afr Med J | year= 2016 | volume= 25 | issue= | pages= 199 | pmid=28270903 | doi=10.11604/pamj.2016.25.199.10758 | pmc=5326263 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=28270903 }} </ref> | |||
</gallery> | </gallery> | ||
==Microscopic Pathology== | ==Microscopic Pathology== | ||
*Histopathology shows | *Histopathology of testes shows atrophic [[seminiferous tubules]] containing only [[sertoli cells]], associated to a [[leydig cells]] [[hyperplasia]].<ref name="pmid26301004">{{cite journal| author=Lachiri B, Hakimi I, Boudhas A, Guelzim K, Kouach J, Oukabli M et al.| title=[Complete androgen insensitivity syndrome: report of two cases and review of literature]. | journal=Pan Afr Med J | year= 2015 | volume= 20 | issue= | pages= 400 | pmid=26301004 | doi=10.11604/pamj.2015.20.400.6760 | pmc=4524922 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=26301004 }} </ref> | ||
* | *The well-limited nodule presents as a circumscribed structure with a thin capsule consisting of atrophic servolian tubes with a very small interstitial tissue with rare [[leydig cells]]. This nodule corresponds to a well differentiated tumor with [[sertoli-leydig cells]]. <ref name="pmid28270903">{{cite journal| author=Souhail R, Amine S, Nadia A, Tarik K, Khalid EK, Abdellatif K et al.| title=Complete androgen insensitivity syndrome or testicular feminization: review of literature based on a case report. | journal=Pan Afr Med J | year= 2016 | volume= 25 | issue= | pages= 199 | pmid=28270903 | doi=10.11604/pamj.2016.25.199.10758 | pmc=5326263 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=28270903 }} </ref> | ||
*The gonads on histopathological examination show the following: <ref name="pmid25395750">{{cite journal |vauthors=Bhaskararao G, Himabindu Y, Nayak SR, Sriharibabu M |title=Laparoscopic gonedectomy in a case of complete androgen insensitivity syndrome |journal=J Hum Reprod Sci |volume=7 |issue=3 |pages=221–3 |year=2014 |pmid=25395750 |pmc=4229800 |doi=10.4103/0974-1208.142498 |url=}}</ref> | |||
:*Thickened [[tunica albuginea]] | |||
:*[[Seminiferous tubules]] with primary and secondary spermatogonia and [[sertoli cells]] | |||
:*Intertubular leydig cells were seen along with peritubular fibrosis | |||
<gallery> | <gallery> | ||
Image: AIS_-_Testes_- | Image:AIS_-_Testes_-_atrophy_of_the_seminiferous_tubules_-_Histopathological_aspect,_HE_staining.jpg|Testes - Atrophy of the seminiferous tubules - Histopathological aspect, HE staining by Regragui Souhail et al from The Pan African Medical Journal - ISSN 1937-8688. Pan Afr Med J. 2016;25:199. Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0). <ref name="pmid28270903">{{cite journal| author=Souhail R, Amine S, Nadia A, Tarik K, Khalid EK, Abdellatif K et al.| title=Complete androgen insensitivity syndrome or testicular feminization: review of literature based on a case report. | journal=Pan Afr Med J | year= 2016 | volume= 25 | issue= | pages= 199 | pmid=28270903 | doi=10.11604/pamj.2016.25.199.10758 | pmc=5326263 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=28270903 }} </ref> | ||
Image: AIS_- | |||
Image:AIS_-_testicular_parenchyma.jpg|Testicular parenchyma of lobulated architecture, made of seminiferous tubes of atrophic appearance; these tubes are lined with Sertolia cells, with no obvious signs of spermatogenesis the interstitium is fibrous with rare clusters of Leydig cells by Boutaina Lachiri et al from the Pan African Medical Journal - ISSN 1937-8688. Pan Afr Med J. 2015;20:400. CC BY-NC 3.0, Creative Commons Attribution License (http://creativecommons.org/licenses/by-nc/3.0). <ref name="pmid26301004">{{cite journal| author=Lachiri B, Hakimi I, Boudhas A, Guelzim K, Kouach J, Oukabli M et al.| title=[Complete androgen insensitivity syndrome: report of two cases and review of literature]. | journal=Pan Afr Med J | year= 2015 | volume= 20 | issue= | pages= 400 | pmid=26301004 | doi=10.11604/pamj.2015.20.400.6760 | pmc=4524922 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=26301004 }} </ref> | |||
</gallery> | </gallery> | ||
Latest revision as of 14:04, 19 October 2017
|
Androgen insensitivity syndrome Microchapters |
|
Differentiating Androgen insensitivity syndrome from other Diseases |
|---|
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
Androgen insensitivity syndrome pathophysiology On the Web |
|
American Roentgen Ray Society Images of Androgen insensitivity syndrome pathophysiology |
|
Directions to Hospitals Treating Androgen insensitivity syndrome |
|
Risk calculators and risk factors for Androgen insensitivity syndrome pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Aravind Reddy Kothagadi M.B.B.S[2]
Overview
Androgen insensitivity syndrome is due to hormone resistance which may be due to defective androgen receptor (AR) function by either abnormal androgen receptor (AR) binding, decreased receptor binding, or impaired androgen receptor (AR) binding. AIS is an X linked disorder. The development of androgen insensitivity syndrome is a result of genetic mutations of the androgen receptor (AR) gene located on the chromosome Xq11-12. Associated conditions include primary amenorrhea, infertility and dyspareunia.
Pathophysiology
Pathogenesis
- Androgen resistance may develop during fetal development and after birth.
- The hormone resistance may be due to defective androgen receptor (AR) function by either abnormal androgen receptor (AR) binding, decreased receptor binding, or impaired androgen receptor (AR) binding. [1][2][3]
- A spectrum of phenotypes may be caused due to missense mutations in the androgen receptor (AR) protein. The two important domains of the receptor protein namely DBD and LDB domains are the ones wherein the most frequent missense mutations are found.[4][5]
- The phenotypic variability impacts and translates the degree to which ligand-binding and receptor functions are disrupted by different substitutions.[4][6]
- The genetic background has an influence on the resulting phenotype as a result of the same mutation which may lead to different forms of AIS within a family.[4][7]
- Mutations in the androgen receptor (AR) gene helps as a trusted tool for the diagnosis and molecular subclassification of AIS. The resulting phenotype is affected by the kind of amino acid substitution occurring due to mutation.[4]
- Information regarding the mutation in the androgen receptor (AR) and its functional consequences helps in determining the genotype-phenotype correlation, to improve and better manage the cases of male pseudohermaphroditism pertaining to surgery of the genitalia, gonadectomy and gender assignment. [4]
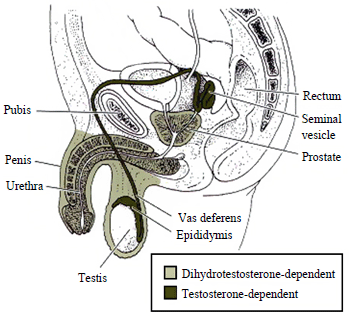
- The lack of virilization of external genitalia is due to the failure of genital folds to fuse and to form scrotum and penis.
- The agenesis of the fallopian tubes, uterus, cervix and proximal vagina is due to the simultaneous testicular production of mullerian inhibiting factor which regresses the mullerian structures.
- In CAIS (complete androgen insensitivity syndrome), the development of the wolffian duct and the differentiation of male external genitalia do not occur correctly, and the Mullerian ducts regress due to the presence of anti-Mullerian hormone (AMH) produced by the sertoli cells of normally developed gonads. The residual Mullerian structures exist in approximately one third of patients. [8] [9]
- The female phenotype along with breast development is a result of the peripheral aromatization of testosterone into estrogen.[10]
- As a result of the defective androgen receptor (AR) due to imperfect feedback mechanism of testosterone at the pituitary and hypothalamus there are elevated levels of serum testosterone, FSH and LH observed.
- Testosterone biosynthetic defects are ruled out by the normal serum 17α hydroxyprogesterone, DHEA and androstenedione levels.[11]
- Sufficient peripheral conversion of testosterone is indicative of normal estradiol level.

Genetics
- AIS is an X linked disorder. [12]
- A high proportion of De novo mutations arise after the zygote stage which occur at a high rate within the androgen receptor (AR) gene.[12]
- Spontaneous mutations in the androgen receptor (AR) gene results in androgen insensitivity syndrome even without any family history.[12]
- The development of androgen insensitivity syndrome is a result of genetic mutations of the androgen receptor (AR) gene located on the chromosome Xq11-12.[4][12]
- Different mutations in the androgen receptor (AR) gene leads to varied clinical phenotypes.[13][14]
- There have been more than 800 mutations in the androgen receptor (AR) gene reported in AIS patients. [15]

Associated Conditions
- Primary amenorrhea
- Infertility
- Dyspareunia
- Common testicular tumors seen in the Androgen insensitivity syndrome are: [16]
- Germ cell tumors
- Sex cord tumors (seminoma)
- Sertoli cell tumors
- Leydig cell tumors
- Hamartomas
Gross Pathology
- Complete androgen insensitivity syndrome in a 30 years old woman presenting with primary amenorrhea.[17]. [18]
-
Front and side view of the patient by Regragui Souhail et al from The Pan African Medical Journal - ISSN 1937-8688. Pan Afr Med J. 2016;25:199. Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0). [17]
-
Clinical aspect of the vagina by Regragui Souhail et al from The Pan African Medical Journal - ISSN 1937-8688. Pan Afr Med J. 2016;25:199. Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0). [17]
-
Intra- abdominal testes - Laparoscopic aspect by Regragui Souhail et al from The Pan African Medical Journal - ISSN 1937-8688. Pan Afr Med J. 2016;25:199. Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0). [17]
-
The excised testis - Macroscopic aspect by Regragui Souhail et al from The Pan African Medical Journal - ISSN 1937-8688. Pan Afr Med J. 2016;25:199. Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0). [17]
Microscopic Pathology
- Histopathology of testes shows atrophic seminiferous tubules containing only sertoli cells, associated to a leydig cells hyperplasia.[18]
- The well-limited nodule presents as a circumscribed structure with a thin capsule consisting of atrophic servolian tubes with a very small interstitial tissue with rare leydig cells. This nodule corresponds to a well differentiated tumor with sertoli-leydig cells. [17]
- The gonads on histopathological examination show the following: [16]
- Thickened tunica albuginea
- Seminiferous tubules with primary and secondary spermatogonia and sertoli cells
- Intertubular leydig cells were seen along with peritubular fibrosis
-
Testes - Atrophy of the seminiferous tubules - Histopathological aspect, HE staining by Regragui Souhail et al from The Pan African Medical Journal - ISSN 1937-8688. Pan Afr Med J. 2016;25:199. Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0). [17]
-
Testicular parenchyma of lobulated architecture, made of seminiferous tubes of atrophic appearance; these tubes are lined with Sertolia cells, with no obvious signs of spermatogenesis the interstitium is fibrous with rare clusters of Leydig cells by Boutaina Lachiri et al from the Pan African Medical Journal - ISSN 1937-8688. Pan Afr Med J. 2015;20:400. CC BY-NC 3.0, Creative Commons Attribution License (http://creativecommons.org/licenses/by-nc/3.0). [18]
References
- ↑ Flier, Jeffrey S.; Underhill, Lisa H.; Griffin, James E. (1992). "Androgen Resistance — The Clinical and Molecular Spectrum". New England Journal of Medicine. 326 (9): 611–618. doi:10.1056/NEJM199202273260906. ISSN 0028-4793.
- ↑ Brown, Terry R.; Maes, Marc; Rothwell, Stephen W.; Migeon, Claude J. (1982). "Human Complete Androgen Insensitivity with Normal Dihydrotestosterone Receptor Binding Capacity in Cultured Genital Skin Fibroblasts: Evidence for a Qualitative Abnormality of the Receptor*". The Journal of Clinical Endocrinology & Metabolism. 55 (1): 61–69. doi:10.1210/jcem-55-1-61. ISSN 0021-972X.
- ↑ Griffin, James E. (1979). "Testicular Feminization Associated with a Thermolabile Androgen Receptor in Cultured Human Fibroblasts". Journal of Clinical Investigation. 64 (6): 1624–1631. doi:10.1172/JCI109624. ISSN 0021-9738.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 Kota SK, Gayatri K, Kota SK, Jammula S (2013). "Genetic analysis of a family with complete androgen insensitivity syndrome". Indian J Hum Genet. 19 (3): 355–7. doi:10.4103/0971-6866.120820. PMC 3841565. PMID 24339553.
- ↑ Brinkmann AO, Faber PW, van Rooij HC, Kuiper GG, Ris C, Klaassen P, van der Korput JA, Voorhorst MM, van Laar JH, Mulder E (1989). "The human androgen receptor: domain structure, genomic organization and regulation of expression". J. Steroid Biochem. 34 (1–6): 307–10. PMID 2626022.
- ↑ McPhaul MJ, Marcelli M, Tilley WD, Griffin JE, Wilson JD (1991). "Androgen resistance caused by mutations in the androgen receptor gene". FASEB J. 5 (14): 2910–5. PMID 1752359.
- ↑ Evans BA, Hughes IA, Bevan CL, Patterson MN, Gregory JW (1997). "Phenotypic diversity in siblings with partial androgen insensitivity syndrome". Arch. Dis. Child. 76 (6): 529–31. PMC 1717223. PMID 9245853.
- ↑ Nichols JL, Bieber EJ, Gell JS (2009). "Case of sisters with complete androgen insensitivity syndrome and discordant Müllerian remnants". Fertil. Steril. 91 (3): 932.e15–8. doi:10.1016/j.fertnstert.2008.09.027. PMID 18930210.
- ↑ Ozdemir O, Sari ME, Akmut E, Selimova V, Unal T, Atalay CR (2014). "Complete androgen insensitivity syndrome with a large gonadal serous papillary cystadenofibroma". J Hum Reprod Sci. 7 (2): 148–50. doi:10.4103/0974-1208.138875. PMC 4150143. PMID 25191030.
- ↑ Hughes IA, Deeb A (2006). "Androgen resistance". Best Pract. Res. Clin. Endocrinol. Metab. 20 (4): 577–98. doi:10.1016/j.beem.2006.11.003. PMID 17161333.
- ↑ Viner RM, Teoh Y, Williams DM, Patterson MN, Hughes IA (1997). "Androgen insensitivity syndrome: a survey of diagnostic procedures and management in the UK". Arch. Dis. Child. 77 (4): 305–9. PMC 1717340. PMID 9389232.
- ↑ 12.0 12.1 12.2 12.3 Akella RR (2017). "Mutational Analysis of Androgen Receptor Gene in Two Families with Androgen Insensitivity". Indian J Endocrinol Metab. 21 (4): 520–523. doi:10.4103/ijem.IJEM_345_16. PMC 5477437. PMID 28670533.
- ↑ Li L, Liu WM, Liu MX, Zheng SQ, Zhang JX, Che FY, Liu SG (2017). "A missense mutation in the androgen receptor gene causing androgen insensitivity syndrome in a Chinese family". Asian J. Androl. 19 (2): 260–261. doi:10.4103/1008-682X.172647. PMC 5312231. PMID 26806084.
- ↑ Brinkmann, Albert O. (2001). "Molecular basis of androgen insensitivity". Molecular and Cellular Endocrinology. 179 (1–2): 105–109. doi:10.1016/S0303-7207(01)00466-X. ISSN 0303-7207.
- ↑ Hughes IA, Werner R, Bunch T, Hiort O (2012). "Androgen insensitivity syndrome". Semin Reprod Med. 30 (5): 432–42. doi:10.1055/s-0032-1324728. PMID 23044881.
- ↑ 16.0 16.1 Bhaskararao G, Himabindu Y, Nayak SR, Sriharibabu M (2014). "Laparoscopic gonedectomy in a case of complete androgen insensitivity syndrome". J Hum Reprod Sci. 7 (3): 221–3. doi:10.4103/0974-1208.142498. PMC 4229800. PMID 25395750.
- ↑ 17.0 17.1 17.2 17.3 17.4 17.5 17.6 Souhail R, Amine S, Nadia A, Tarik K, Khalid EK, Abdellatif K, Ahmed A (2016). "Complete androgen insensitivity syndrome or testicular feminization: review of literature based on a case report". Pan Afr Med J. 25: 199. doi:10.11604/pamj.2016.25.199.10758. PMC 5326263. PMID 28270903.
- ↑ 18.0 18.1 18.2 Lachiri B, Hakimi I, Boudhas A, Guelzim K, Kouach J, Oukabli M, Rahali DM, Dehayni M (2015). "[Complete androgen insensitivity syndrome: report of two cases and review of literature]". Pan Afr Med J (in French). 20: 400. doi:10.11604/pamj.2015.20.400.6760. PMC 4524922. PMID 26301004.
![Front and side view of the patient by Regragui Souhail et al from The Pan African Medical Journal - ISSN 1937-8688. Pan Afr Med J. 2016;25:199. Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0). [17]](/images/e/e3/AIS_-_Front_and_side_view_of_the_patient.jpg)
![Clinical aspect of the vagina by Regragui Souhail et al from The Pan African Medical Journal - ISSN 1937-8688. Pan Afr Med J. 2016;25:199. Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0). [17]](/images/e/eb/AIS_-_Clinical_aspect_of_the_vagina.jpg)
![Intra- abdominal testes - Laparoscopic aspect by Regragui Souhail et al from The Pan African Medical Journal - ISSN 1937-8688. Pan Afr Med J. 2016;25:199. Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0). [17]](/images/d/d8/AIS_-_Intra-_abdominal_testes_-_laparoscopic_aspect.jpg)
![The excised testis - Macroscopic aspect by Regragui Souhail et al from The Pan African Medical Journal - ISSN 1937-8688. Pan Afr Med J. 2016;25:199. Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0). [17]](/images/7/7d/AIS_-_The_excised_testis_-_Macroscopic_aspect.jpg)
![Testes - Atrophy of the seminiferous tubules - Histopathological aspect, HE staining by Regragui Souhail et al from The Pan African Medical Journal - ISSN 1937-8688. Pan Afr Med J. 2016;25:199. Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0). [17]](/images/b/b5/AIS_-_Testes_-_atrophy_of_the_seminiferous_tubules_-_Histopathological_aspect%2C_HE_staining.jpg)
![Testicular parenchyma of lobulated architecture, made of seminiferous tubes of atrophic appearance; these tubes are lined with Sertolia cells, with no obvious signs of spermatogenesis the interstitium is fibrous with rare clusters of Leydig cells by Boutaina Lachiri et al from the Pan African Medical Journal - ISSN 1937-8688. Pan Afr Med J. 2015;20:400. CC BY-NC 3.0, Creative Commons Attribution License (http://creativecommons.org/licenses/by-nc/3.0). [18]](/images/8/8a/AIS_-_testicular_parenchyma.jpg)