Aminocaproic acid (oral): Difference between revisions
No edit summary |
m (Protected "Aminocaproic acid (oral)": Bot: Protecting all pages from category Drug ([Edit=Allow only administrators] (indefinite) [Move=Allow only administrators] (indefinite))) |
||
| (12 intermediate revisions by 3 users not shown) | |||
| Line 5: | Line 5: | ||
|drugClass=[[hemostatic agent]] | |drugClass=[[hemostatic agent]] | ||
|indicationType=treatment | |indicationType=treatment | ||
|indication=surgical complications following heart surgery (with or without cardiac bypass procedures) and portacaval shunt; hematological disorders such as amegakaryocytic thrombocytopenia (accompanying aplastic anemia); acute and life-threatening abruptio placentae; hepatic cirrhosis; and neoplastic disease such as carcinoma of the prostate, lung, stomach, and cervix | |indication=surgical complications following [[heart]] surgery (with or without [[cardiac bypass]] procedures) and [[portacaval shunt]]; hematological disorders such as [[amegakaryocytic thrombocytopenia]] (accompanying [[aplastic anemia]]); acute and life-threatening [[abruptio placentae]]; [[hepatic cirrhosis]]; and [[neoplastic disease]] such as [[carcinoma]] of the [[prostate]], [[lung]], [[stomach]], and [[cervix]] | ||
|adverseReactions=[[abdominal pain]], [[nausea]], [[vomiting]], [[diarrhea]], [[headache]] [[dizziness]], [[confusion]], [[hallucinations]], blurred vision, [[tinnitus]] | |adverseReactions=[[abdominal pain]], [[nausea]], [[vomiting]], [[diarrhea]], [[headache]] [[dizziness]], [[confusion]], [[hallucinations]], [[blurred vision]], and [[tinnitus]] | ||
|blackBoxWarningTitle=<span style="color:#FF0000;">ConditionName: </span> | |blackBoxWarningTitle=<span style="color:#FF0000;">ConditionName: </span> | ||
|blackBoxWarningBody=<i><span style="color:#FF0000;">ConditionName: </span></i> | |blackBoxWarningBody=<i><span style="color:#FF0000;">ConditionName: </span></i> | ||
| Line 16: | Line 16: | ||
<!--FDA-Labeled Indications and Dosage (Adult)--> | <!--FDA-Labeled Indications and Dosage (Adult)--> | ||
|fdaLIADAdult======Indication===== | |fdaLIADAdult======Indication===== | ||
AMICAR is useful in enhancing hemostasis when fibrinolysis contributes to bleeding. In life-threatening situations, transfusion of appropriate blood products and other emergency measures may be required. | * AMICAR is useful in enhancing [[hemostasis]] when [[fibrinolysis]] contributes to bleeding. In life-threatening situations, [[transfusion]] of appropriate blood products and other [[emergency]] measures may be required. | ||
* [[Fibrinolytic]] bleeding may frequently be associated with surgical complications following [[heart surgery]] (with or without [[cardiac bypass]] procedures) and [[portacaval shunt]]; [[hematological disorders]] such as [[amegakaryocytic]] [[thrombocytopenia]] (accompanying [[aplastic anemia]]); acute and life-threatening [[abruptio placentae]]; [[hepatic cirrhosis]]; and [[neoplastic disease]] such as [[carcinoma of the prostate]], [[lung]], [[stomach]], and [[cervix]]. | |||
Fibrinolytic bleeding may frequently be associated with surgical complications following heart surgery (with or without cardiac bypass procedures) and portacaval shunt; hematological disorders such as amegakaryocytic thrombocytopenia (accompanying aplastic anemia); acute and life-threatening abruptio placentae; hepatic cirrhosis; and neoplastic disease such as carcinoma of the prostate, lung, stomach, and cervix. | * [[Urinary fibrinolysis]], usualIy a normal physiological phenomenon, may contribute to excessive urinary tract fibrinolytic bleeding associated with surgical [[hematuria]] (following [[prostatectomy]] and [[nephrectomy]]) or nonsurgical [[hematuria]] (accompanying [[polycystic]] or [[neoplastic diseases]] of the [[genitourinary system]]) | ||
Urinary fibrinolysis, usualIy a normal physiological phenomenon, may contribute to excessive urinary tract fibrinolytic bleeding associated with surgical hematuria (following prostatectomy and nephrectomy) or nonsurgical hematuria (accompanying polycystic or neoplastic diseases of the genitourinary system) | |||
=====Dosage===== | =====Dosage===== | ||
An identical dosage regimen may be followed by administering | * An identical dosage regimen may be followed by administering aminocaproic acid tablets or AMICAR oral solution as follows: | ||
* For the treatment of acute bleeding syndromes due to elevated fibrinolytic activity, it is suggested that 5 aminocaproic acid 1000 mg tablets or 10 aminocaproic acid 500 mg tablets (5 g) or 20 milliliter of aminocaproic acid oral solution (5 g) be administered during the first hour of treatment, followed by a continuing rate of 1 aminocaproic acid 1000 mg Tablet or 2 aminocaproic acid 500 mg tablets (1 g) or 5 milliliter of aminocaproic acid oral solution (1.25 g) per hour. This method of treatment would ordinarily be continued for about 8 hours or until the bleeding situation has been controlled. | |||
For the treatment of acute bleeding syndromes due to elevated fibrinolytic activity, it is suggested that 5 | |offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | ||
|offLabelAdultGuideSupport= | |||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
<!--Non–Guideline-Supported Use (Adult)--> | <!--Non–Guideline-Supported Use (Adult)--> | ||
|offLabelAdultNoGuideSupport= | |offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | ||
There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
<!--Pediatric Indications and Dosage--> | <!--Pediatric Indications and Dosage--> | ||
<!--FDA-Labeled Indications and Dosage (Pediatric)--> | <!--FDA-Labeled Indications and Dosage (Pediatric)--> | ||
|fdaLIADPed= | |fdaLIADPed=There is limited information regarding <i>FDA-Labeled Use</i> of {{PAGENAME}} in pediatric patients. | ||
There is limited information regarding <i>FDA-Labeled Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Off-Label Use and Dosage (Pediatric)--> | <!--Off-Label Use and Dosage (Pediatric)--> | ||
<!--Guideline-Supported Use (Pediatric)--> | <!--Guideline-Supported Use (Pediatric)--> | ||
|offLabelPedGuideSupport= | |offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | ||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Non–Guideline-Supported Use (Pediatric)--> | <!--Non–Guideline-Supported Use (Pediatric)--> | ||
|offLabelPedNoGuideSupport= | |offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | ||
There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Contraindications--> | <!--Contraindications--> | ||
|contraindications=* | |contraindications=* Aminocaproic acid should not be used when there is evidence of an active intravascular clotting process. | ||
* When there is uncertainty as to whether the cause of bleeding is primary fibrinolysis or [[disseminated intravascular coagulation]] (DIC), this distinction must be made before administering aminocaproic acid. | |||
When there is uncertainty as to whether the cause of bleeding is primary fibrinolysis or disseminated intravascular coagulation (DIC), this distinction must be made before administering | * The following tests can be applied to differentiate the two conditions: | ||
* [[Platelet count]] is usually decreased in DIC but normal in primary [[fibrinolysis]]. | |||
The following tests can be applied to differentiate the two conditions: | * [[Protamine paracoagulation test]] is positive in [[DIC]]; a precipitate forms when [[protamine sulfate]] is dropped into [[citrated plasma]]. The test is negative in the presence of primary [[fibrinolysis]]. | ||
* The euglobulin clot Iysis test is abnormal in primary [[fibrinolysis]] but normal in [[DIC]]. | |||
Platelet count is usually decreased in DIC but normal in primary fibrinolysis. | * Aminocaproic acid must not be used in the presence of [[DIC]] without concomitant [[heparin]]. | ||
|warnings=* In patients with upper [[urinary tract bleeding]], aminocaproic acid administration has been known to cause [[intrarenal obstruction]] in the form of [[glomerular capillary thrombosis]] or clots in the [[renal pelvis]] and [[ureters]]. For this reason, aminocaproic acid should not be used in [[hematuria]] of upper urinary tract origin, unless the possible benefits outweigh the risk. | |||
Protamine paracoagulation test is positive in DIC; a precipitate forms when protamine sulfate is dropped into citrated plasma. The test is negative in the presence of primary fibrinolysis. | * [[Subendocardial hemorrhages]] have been observed in dogs given [[intravenous]] infusions of 0.2 times the maximum human therapeutic dose of aminocaproic acid and in monkeys given 8 times the maximum human therapeutic dose of aminocaproic acid. | ||
* Fatty degeneration of the [[myocardium]] has been reported in dogs given [[intravenous]] doses of aminocaproic acid at 0.8 to 3.3 times the maximum human therapeutic dose and in monkeys given [[intravenous]] doses of aminocaproic acid at 6 times the maximum human therapeutic dose. | |||
The euglobulin clot Iysis test is abnormal in primary fibrinolysis but normal in DIC. | * Rarely, [[skeletal muscle]] weakness with necrosis of muscle fibers has been reported following prolonged administration. Clinical presentation may range from mild [[myalgias]] with weakness and [[fatigue]] to a severe [[proximal myopathy]] with [[rhabdomyolysis]], [[myoglobinuria]], and [[acute renal failure]]. [[Muscle enzymes]], especially [[creatine phosphokinase]] (CPK) are elevated. [[CPK]] levels should be monitored in patients on long-term therapy. Aminocaproic acid administration should be stopped if a rise in [[CPK]] is noted. Resolution follows discontinuation of aminocaproic acid; however, the syndrome may recur if aminocaproic acid is restarted. | ||
* The possibility of [[cardiac muscle]] damage should also be considered when [[skeletal myopathy]] occurs. One case of cardiac and hepatic lesions observed in man has been reported. The patient received 2 g of aminocaproic acid every 6 hours for a total dose of 26 g. Death was due to continued [[cerebrovascular hemorrhage]]. Necrotic changes in the [[heart]] and [[liver]] were noted at [[autopsy]]. | |||
|warnings=* In patients with upper urinary tract bleeding, | |||
Subendocardial hemorrhages have been observed in dogs given intravenous infusions of 0.2 times the maximum human therapeutic dose of | |||
Fatty degeneration of the myocardium has been reported in dogs given intravenous doses of | |||
Rarely, skeletal muscle weakness with necrosis of muscle fibers has been reported following prolonged administration. Clinical presentation may range from mild myalgias with weakness and fatigue to a severe proximal myopathy with rhabdomyolysis, myoglobinuria, and acute renal failure. Muscle enzymes, especially creatine phosphokinase (CPK) are elevated. CPK levels should be monitored in patients on long-term therapy. | |||
The possibility of cardiac muscle damage should also be considered when skeletal myopathy occurs. One case of cardiac and hepatic lesions observed in man has been reported. The patient received 2 g of aminocaproic acid every 6 hours for a total dose of 26 g. Death was due to continued cerebrovascular hemorrhage. Necrotic changes in the heart and liver were noted at autopsy. | |||
|clinicalTrials=There is limited information regarding <i>Clinical Trial Experience</i> of {{PAGENAME}} in the drug label. | |clinicalTrials=There is limited information regarding <i>Clinical Trial Experience</i> of {{PAGENAME}} in the drug label. | ||
|postmarketing=* Aminocaproic acid is generally well tolerated. The following adverse experiences have been reported: | |||
:* General: Edema, headache, malaise. | |||
|postmarketing= | :* Hypersensitivity Reactions: Allergic and anaphylactoid reactions, anaphylaxis. | ||
:* Cardiovascular: Bradycardia, hypotension, peripheral ischemia, thrombosis. | |||
General: Edema, headache, malaise. | :* Gastrointestinal: Abdominal pain, diarrhea, nausea, vomiting. | ||
:* Hematologic: Agranulocytosis, coagulation disorder, leukopenia, thrombocytopenia. | |||
Hypersensitivity Reactions: Allergic and anaphylactoid reactions, anaphylaxis. | :* Musculoskeletal: CPK increased, muscle weakness, myalgia, myopathy (see WARNINGS), myositis, rhabdomyolysis. | ||
:* Neurologic: Confusion, convulsions, delirium, dizziness, hallucinations, intracranial hypertension, stroke, syncope. | |||
Cardiovascular: Bradycardia, hypotension, peripheral ischemia, thrombosis. | :* Respiratory: Dyspnea, nasal congestion, pulmonary embolism. | ||
:* Skin: Pruritis, rash. | |||
Gastrointestinal: Abdominal pain, diarrhea, nausea, vomiting. | :* Special Senses: Tinnitus, vision decreased, watery eyes. | ||
:* Urogenital: BUN increased, renal failure. There have been some reports of dry ejaculation during the period of aminocaproic acid treatment. These have been reported to date only in hemophilia patients who received the drug after undergoing dental surgical procedures. However, this symptom resolved in all patients within 24 to 48 hours of completion of therapy. | |||
Hematologic: Agranulocytosis, coagulation disorder, leukopenia, thrombocytopenia. | |drugInteractions=<!--Use in Specific Populations--> | ||
Musculoskeletal: CPK increased, muscle weakness, myalgia, myopathy (see WARNINGS), myositis, rhabdomyolysis. | |||
Neurologic: Confusion, convulsions, delirium, dizziness, hallucinations, intracranial hypertension, stroke, syncope. | |||
Respiratory: Dyspnea, nasal congestion, pulmonary embolism. | |||
Skin: Pruritis, rash. | |||
Special Senses: Tinnitus, vision decreased, watery eyes. | |||
Urogenital: BUN increased, renal failure. There have been some reports of dry ejaculation during the period of | |||
|drugInteractions= | |||
<!--Use in Specific Populations--> | |||
|FDAPregCat=C | |FDAPregCat=C | ||
|useInPregnancyFDA=* Animal reproduction studies have not been conducted with | |useInPregnancyFDA=* Animal reproduction studies have not been conducted with aminocaproic acid. It is also not known whether aminocaproic acid can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. aminocaproic acid should be given to a pregnant woman only if clearly needed. | ||
|useInPregnancyAUS=* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | |useInPregnancyAUS=* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | ||
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | ||
|useInLaborDelivery=There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | |useInLaborDelivery=There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | ||
|useInNursing=It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when | |useInNursing=* It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when aminocaproic acid is administered to a nursing woman. | ||
|useInPed=There is no FDA guidance on the use of {{PAGENAME}} with respect to pediatric patients. | |useInPed=There is no FDA guidance on the use of {{PAGENAME}} with respect to pediatric patients. | ||
|useInGeri=There is no FDA guidance on the use of {{PAGENAME}} with respect to geriatric patients. | |useInGeri=There is no FDA guidance on the use of {{PAGENAME}} with respect to geriatric patients. | ||
| Line 120: | Line 84: | ||
<!--Administration and Monitoring--> | <!--Administration and Monitoring--> | ||
|administration=* Oral | |administration=* [[Oral]] | ||
|monitoring=* Muscle enzymes, especially creatine phosphokinase (CPK) are elevated. CPK levels should be monitored in patients on long-term therapy. | |||
|IVCompat=There is limited information regarding <i>IV Compatibility</i> of {{PAGENAME}} in the drug label. | |||
<!--Overdosage--> | |||
|overdose=* A few cases of acute overdosage with aminocaproic acid administered intravenously have been reported. The effects have ranged from no reaction to transient hypotension to severe acute renal failure leading to death. One patient with a history of brain tumor and seizures experienced seizures after receiving an 8 gram bolus injection of aminocaproic acid. | |||
* The single dose of aminocaproic acid causing symptoms of overdosage or considered to be life-threatening is unknown. Patients have tolerated doses as high as 100 grams while acute renal failure has been reported following a dose of 12 grams. | |||
* The intravenous and oral LD50 of aminocaproic acid were 3.0 and 12.0 g/kg, respectively, in the mouse and 3.2 and 16.4 g/kg, respectively, in the rat. An intravenous infusion dose of 2.3 g/kg was lethal in the dog. On intravenous administration, tonic-clonic convulsions were observed in dogs and mice. | |||
* No treatment for overdosage is known, although evidence exists that aminocaproic acid is removed by hemodialysis and may be removed by peritoneal dialysis. Pharmacokinetic studies have shown that total body clearance of aminocaproic acid is markedly decreased in patients with severe renal failure. | |||
|drugBox={{Drugbox2 | |||
| verifiedrevid = 476999776 | |||
| IUPAC_name = 6-aminohexanoic acid | |||
| image = Aminocaproic01.jpg | |||
| | <!--Clinical data--> | ||
| | | tradename = aminocaproic acid | ||
| Drugs.com = {{drugs.com|monograph|aminocaproic_acid}} | |||
| MedlinePlus = a608023 | |||
| pregnancy_category = | |||
| legal_status = Rx-only | |||
| routes_of_administration = | |||
<!-- | <!--Pharmacokinetic data--> | ||
| | | bioavailability = | ||
| protein_bound = | |||
| metabolism = [[Renal]] | |||
| elimination_half-life = 2 hours | |||
<!--Identifiers--> | |||
| CASNo_Ref = {{cascite|correct|CAS}} | |||
| CAS_number_Ref = {{cascite|correct|??}} | |||
| CAS_number = 60-32-2 | |||
| ATC_prefix = B02 | |||
| ATC_suffix = AA01 | |||
| ATC_supplemental = | |||
| PubChem = 564 | |||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| DrugBank = DB00513 | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 548 | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = U6F3787206 | |||
| KEGG_Ref = {{keggcite|correct|kegg}} | |||
| KEGG = D00160 | |||
| ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| ChEBI = 16586 | |||
| ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| ChEMBL = 1046 | |||
| NIAID_ChemDB = 018631 | |||
<!--Chemical data--> | |||
| | | C=6 | H=13 | N=1 | O=2 | ||
| molecular_weight = 131.173 g/mol | |||
| smiles = O=C(O)C(N)CCCC | |||
| InChI = 1/C6H13NO2/c1-2-3-4-5(7)6(8)9/h5H,2-4,7H2,1H3,(H,8,9) | |||
| InChIKey = LRQKBLKVPFOOQJ-UHFFFAOYAT | |||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChI = 1S/C6H13NO2/c7-5-3-1-2-4-6(8)9/h1-5,7H2,(H,8,9) | |||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChIKey = SLXKOJJOQWFEFD-UHFFFAOYSA-N | |||
}} | |||
|mechAction=* | |mechAction=* | ||
<!--Structure--> | <!--Structure--> | ||
|structure=: [[File: | |structure=: [[File:Structure amino cap.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
* Aminocaproic acid is soluble in water, acid, and alkaline solutions; it is sparingly soluble in methanol and practically insoluble in chloroform. | |||
* aminocaproic acid oral solution for oral administration, contains 0.25 g/mL of aminocaproic acid with methylparaben 0.20%, propylparaben 0.05%, edetate disodium 0.30% as preservatives and the following inactive ingredients: sodium saccharin, sorbitol solution, citric acid anhydrous, natural and artificial raspberry flavor and an artificial bitterness modifier. | |||
* Each aminocaproic acid (aminocaproic acid) Tablet, for oral administration contains 500 mg or 1000 mg of aminocaproic acid and the following inactive ingredients: povidone, crospovidone, stearic acid, and magnesium stearate. | |||
Each | |||
| Line 149: | Line 158: | ||
<!--Pharmacokinetics--> | <!--Pharmacokinetics--> | ||
|PK=The fibrinolysis-inhibitory effects of | |PK=* The fibrinolysis-inhibitory effects of aminocaproic acid appear to be exerted principally via inhibition of plasminogen activators and to a lesser degree through antiplasmin activity. | ||
* In adults, oral absorption appears to be a zero-order process with an absorption rate of 5.2 g/hr. The mean lag time in absorption is 10 minutes. After a single oral dose of 5 g, absorption was complete (F=1). Mean ± SD peak plasma concentrations (164 ± 28 mcg/mL) were reached within 1.2 ± 0.45 hours. | |||
In adults, oral absorption appears to be a zero-order process with an absorption rate of 5.2 g/hr. The mean lag time in absorption is 10 minutes. After a single oral dose of 5 g, absorption was complete (F=1). Mean ± SD peak plasma concentrations (164 ± 28 mcg/mL) were reached within 1.2 ± 0.45 hours. | * After oral administration, the apparent volume of distribution was estimated to be 23.1 ± 6.6 L (mean ± SD). Correspondingly, the volume of distribution after intravenous administration has been reported to be 30.0 ± 8.2 L. After prolonged administration, aminocaproic acid has been found to distribute throughout extravascular and intravascular compartments of the body, penetrating human red blood cells as well as other tissue cells. | ||
* Renal excretion is the primary route of elimination. Sixty-five percent of the dose is recovered in the urine as unchanged drug and 11% of the dose appears as the metabolite adipic acid. Renal clearance (116 mL/min) approximates endogenous creatinine clearance. The total body clearance is 169 mL/min. The terminal elimination half-life for aminocaproic acid is approximately 2 hours. | |||
After oral administration, the apparent volume of distribution was estimated to be 23.1 ± 6.6 L (mean ± SD). Correspondingly, the volume of distribution after intravenous administration has been reported to be 30.0 ± 8.2 L. After prolonged administration, | |||
Renal excretion is the primary route of elimination. Sixty-five percent of the dose is recovered in the urine as unchanged drug and 11% of the dose appears as the metabolite adipic acid. Renal clearance (116 mL/min) approximates endogenous creatinine clearance. The total body clearance is 169 mL/min. The terminal elimination half-life for | |||
|nonClinToxic=There is limited information regarding <i>Nonclinical Toxicology</i> of {{PAGENAME}} in the drug label. | |nonClinToxic=There is limited information regarding <i>Nonclinical Toxicology</i> of {{PAGENAME}} in the drug label. | ||
| Line 162: | Line 168: | ||
<!--How Supplied--> | <!--How Supplied--> | ||
|howSupplied=* | |howSupplied= | ||
* Aminocaproic acid oral solution, 0.25 g/mL | |||
* Each mL of raspberry-flavored oral solution contains 0.25 g/mL of aminocaproic acid. | |||
:* 16 Fl. Oz. (473 mL) Bottle – NDC 49411-052-16 | |||
* AMICAR 500 mg Tablets | |||
:* Each round, white tablet, engraved with XP on one side and scored on the other with A to the left of the score and 10 on the right, contains 500 mg of aminocaproic acid. | |||
Each mL of raspberry-flavored oral solution contains 0.25 g/mL of aminocaproic acid. | :* Bottle of 100 – NDC 49411-050-01 | ||
* AMICAR 1000 mg Tablets | |||
16 Fl. Oz. (473 mL) Bottle – NDC 49411-052-16 | :* Each oblong, white tablet, engraved with XP on one side and scored on the other with A to the left of the score and 20 on the right, contains 1000 mg of aminocaproic acid. | ||
:* Bottle of 100 – NDC 49411-051-01 | |||
|storage=* Store at 20°-25°C (68°-77°F); Dispense in Tight Containers; Do Not Freeze. | |||
|packLabel=[[File:Am 01.jpg|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
AMICAR 500 mg Tablets | [[File:Am 02 0.jpg|thumb|none|400px|This image is provided by the National Library of Medicine.]] | ||
[[File:Am 03.jpg|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
Each round, white tablet, engraved with XP on one side and scored on the other with A to the left of the score and 10 on the right, contains 500 mg of aminocaproic acid. | [[File:DailyMed AMICAR aminocaproic acid solution AMICAR aminocaproic acid tablet.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | ||
Bottle of 100 – NDC 49411-050-01 | |||
AMICAR 1000 mg Tablets | |||
Each oblong, white tablet, engraved with XP on one side and scored on the other with A to the left of the score and 20 on the right, contains 1000 mg of aminocaproic acid. | |||
Bottle of 100 – NDC 49411-051-01 | |||
|storage=* Store at 20°-25°C (68°-77°F) | |||
|packLabel=[[File: | |||
[[File: | |||
[[File: | |||
|fdaPatientInfo=There is limited information regarding <i>Patient Counseling Information</i> of {{PAGENAME}} in the drug label. | |fdaPatientInfo=There is limited information regarding <i>Patient Counseling Information</i> of {{PAGENAME}} in the drug label. | ||
| Line 198: | Line 190: | ||
<!--Brand Names--> | <!--Brand Names--> | ||
|brandNames=* AMICAR®<ref>{{Cite web | title =AMICAR- aminocaproic acid solution | url =http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2238c70f-b0b5-4755-896b-45b28777b217 }}</ref> | |brandNames=* AMICAR®<ref>{{Cite web | title =AMICAR- aminocaproic acid solution | url =http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2238c70f-b0b5-4755-896b-45b28777b217 }}</ref> | ||
|drugShortage= | |drugShortage= | ||
}} | }} | ||
{{PillImage | {{PillImage|fileName=Aminocaproic_Acid_NDC_617480045.jpg|drugName=Aminocaproic Acid|NDC=617480045|drugAuthor=VersaPharm Incorporated|ingredients=AMINOCAPROIC ACID[AMINOCAPROIC ACID]|pillImprint=VP;045|dosageValue=500|dosageUnit=mg|pillColor=White|pillShape=Round|pillSize=13|pillScore=2}} | ||
|fileName= | {{PillImage|fileName=Amicar_NDC_664790021.jpg|drugName=Amicar|NDC=664790021|drugAuthor=Xanodyne Pharmaceuticals, Inc.|ingredients=Aminocaproic Acid[Aminocaproic Acid]|pillImprint=XP;A;10|dosageValue=500|dosageUnit=mg|pillColor=White|pillShape=Round|pillSize=13|pillScore=2}} | ||
}} | |||
{{ | |||
|fileName= | |||
| | |||
}} | |||
<!--Label Display Image--> | <!--Label Display Image--> | ||
<!--Category--> | <!--Category--> | ||
[[Category:Drug]] | [[Category:Drug]] | ||
[[Category:Amino acids]] | |||
[[Category:Antifibrinolytics]] | |||
Latest revision as of 17:29, 18 August 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ammu Susheela, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Aminocaproic acid (oral) is a hemostatic agent that is FDA approved for the treatment of surgical complications following heart surgery (with or without cardiac bypass procedures) and portacaval shunt; hematological disorders such as amegakaryocytic thrombocytopenia (accompanying aplastic anemia); acute and life-threatening abruptio placentae; hepatic cirrhosis; and neoplastic disease such as carcinoma of the prostate, lung, stomach, and cervix. Common adverse reactions include abdominal pain, nausea, vomiting, diarrhea, headache dizziness, confusion, hallucinations, blurred vision, and tinnitus.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indication
- AMICAR is useful in enhancing hemostasis when fibrinolysis contributes to bleeding. In life-threatening situations, transfusion of appropriate blood products and other emergency measures may be required.
- Fibrinolytic bleeding may frequently be associated with surgical complications following heart surgery (with or without cardiac bypass procedures) and portacaval shunt; hematological disorders such as amegakaryocytic thrombocytopenia (accompanying aplastic anemia); acute and life-threatening abruptio placentae; hepatic cirrhosis; and neoplastic disease such as carcinoma of the prostate, lung, stomach, and cervix.
- Urinary fibrinolysis, usualIy a normal physiological phenomenon, may contribute to excessive urinary tract fibrinolytic bleeding associated with surgical hematuria (following prostatectomy and nephrectomy) or nonsurgical hematuria (accompanying polycystic or neoplastic diseases of the genitourinary system)
Dosage
- An identical dosage regimen may be followed by administering aminocaproic acid tablets or AMICAR oral solution as follows:
- For the treatment of acute bleeding syndromes due to elevated fibrinolytic activity, it is suggested that 5 aminocaproic acid 1000 mg tablets or 10 aminocaproic acid 500 mg tablets (5 g) or 20 milliliter of aminocaproic acid oral solution (5 g) be administered during the first hour of treatment, followed by a continuing rate of 1 aminocaproic acid 1000 mg Tablet or 2 aminocaproic acid 500 mg tablets (1 g) or 5 milliliter of aminocaproic acid oral solution (1.25 g) per hour. This method of treatment would ordinarily be continued for about 8 hours or until the bleeding situation has been controlled.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Aminocaproic acid (oral) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Aminocaproic acid (oral) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Aminocaproic acid (oral) in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Aminocaproic acid (oral) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Aminocaproic acid (oral) in pediatric patients.
Contraindications
- Aminocaproic acid should not be used when there is evidence of an active intravascular clotting process.
- When there is uncertainty as to whether the cause of bleeding is primary fibrinolysis or disseminated intravascular coagulation (DIC), this distinction must be made before administering aminocaproic acid.
- The following tests can be applied to differentiate the two conditions:
- Platelet count is usually decreased in DIC but normal in primary fibrinolysis.
- Protamine paracoagulation test is positive in DIC; a precipitate forms when protamine sulfate is dropped into citrated plasma. The test is negative in the presence of primary fibrinolysis.
- The euglobulin clot Iysis test is abnormal in primary fibrinolysis but normal in DIC.
- Aminocaproic acid must not be used in the presence of DIC without concomitant heparin.
Warnings
- In patients with upper urinary tract bleeding, aminocaproic acid administration has been known to cause intrarenal obstruction in the form of glomerular capillary thrombosis or clots in the renal pelvis and ureters. For this reason, aminocaproic acid should not be used in hematuria of upper urinary tract origin, unless the possible benefits outweigh the risk.
- Subendocardial hemorrhages have been observed in dogs given intravenous infusions of 0.2 times the maximum human therapeutic dose of aminocaproic acid and in monkeys given 8 times the maximum human therapeutic dose of aminocaproic acid.
- Fatty degeneration of the myocardium has been reported in dogs given intravenous doses of aminocaproic acid at 0.8 to 3.3 times the maximum human therapeutic dose and in monkeys given intravenous doses of aminocaproic acid at 6 times the maximum human therapeutic dose.
- Rarely, skeletal muscle weakness with necrosis of muscle fibers has been reported following prolonged administration. Clinical presentation may range from mild myalgias with weakness and fatigue to a severe proximal myopathy with rhabdomyolysis, myoglobinuria, and acute renal failure. Muscle enzymes, especially creatine phosphokinase (CPK) are elevated. CPK levels should be monitored in patients on long-term therapy. Aminocaproic acid administration should be stopped if a rise in CPK is noted. Resolution follows discontinuation of aminocaproic acid; however, the syndrome may recur if aminocaproic acid is restarted.
- The possibility of cardiac muscle damage should also be considered when skeletal myopathy occurs. One case of cardiac and hepatic lesions observed in man has been reported. The patient received 2 g of aminocaproic acid every 6 hours for a total dose of 26 g. Death was due to continued cerebrovascular hemorrhage. Necrotic changes in the heart and liver were noted at autopsy.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Clinical Trial Experience of Aminocaproic acid (oral) in the drug label.
Postmarketing Experience
- Aminocaproic acid is generally well tolerated. The following adverse experiences have been reported:
- General: Edema, headache, malaise.
- Hypersensitivity Reactions: Allergic and anaphylactoid reactions, anaphylaxis.
- Cardiovascular: Bradycardia, hypotension, peripheral ischemia, thrombosis.
- Gastrointestinal: Abdominal pain, diarrhea, nausea, vomiting.
- Hematologic: Agranulocytosis, coagulation disorder, leukopenia, thrombocytopenia.
- Musculoskeletal: CPK increased, muscle weakness, myalgia, myopathy (see WARNINGS), myositis, rhabdomyolysis.
- Neurologic: Confusion, convulsions, delirium, dizziness, hallucinations, intracranial hypertension, stroke, syncope.
- Respiratory: Dyspnea, nasal congestion, pulmonary embolism.
- Skin: Pruritis, rash.
- Special Senses: Tinnitus, vision decreased, watery eyes.
- Urogenital: BUN increased, renal failure. There have been some reports of dry ejaculation during the period of aminocaproic acid treatment. These have been reported to date only in hemophilia patients who received the drug after undergoing dental surgical procedures. However, this symptom resolved in all patients within 24 to 48 hours of completion of therapy.
Drug Interactions
There is limited information regarding Aminocaproic acid (oral) Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- Animal reproduction studies have not been conducted with aminocaproic acid. It is also not known whether aminocaproic acid can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. aminocaproic acid should be given to a pregnant woman only if clearly needed.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Aminocaproic acid (oral) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Aminocaproic acid (oral) during labor and delivery.
Nursing Mothers
- It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when aminocaproic acid is administered to a nursing woman.
Pediatric Use
There is no FDA guidance on the use of Aminocaproic acid (oral) with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Aminocaproic acid (oral) with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Aminocaproic acid (oral) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Aminocaproic acid (oral) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Aminocaproic acid (oral) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Aminocaproic acid (oral) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Aminocaproic acid (oral) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Aminocaproic acid (oral) in patients who are immunocompromised.
Administration and Monitoring
Administration
Monitoring
- Muscle enzymes, especially creatine phosphokinase (CPK) are elevated. CPK levels should be monitored in patients on long-term therapy.
IV Compatibility
There is limited information regarding IV Compatibility of Aminocaproic acid (oral) in the drug label.
Overdosage
- A few cases of acute overdosage with aminocaproic acid administered intravenously have been reported. The effects have ranged from no reaction to transient hypotension to severe acute renal failure leading to death. One patient with a history of brain tumor and seizures experienced seizures after receiving an 8 gram bolus injection of aminocaproic acid.
- The single dose of aminocaproic acid causing symptoms of overdosage or considered to be life-threatening is unknown. Patients have tolerated doses as high as 100 grams while acute renal failure has been reported following a dose of 12 grams.
- The intravenous and oral LD50 of aminocaproic acid were 3.0 and 12.0 g/kg, respectively, in the mouse and 3.2 and 16.4 g/kg, respectively, in the rat. An intravenous infusion dose of 2.3 g/kg was lethal in the dog. On intravenous administration, tonic-clonic convulsions were observed in dogs and mice.
- No treatment for overdosage is known, although evidence exists that aminocaproic acid is removed by hemodialysis and may be removed by peritoneal dialysis. Pharmacokinetic studies have shown that total body clearance of aminocaproic acid is markedly decreased in patients with severe renal failure.
Pharmacology
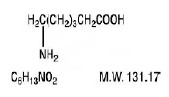
| |
Aminocaproic acid (oral)
| |
| Systematic (IUPAC) name | |
| 6-aminohexanoic acid | |
| Identifiers | |
| CAS number | |
| ATC code | B02 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 131.173 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | Renal |
| Half life | 2 hours |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status |
Template:Unicode Prescription only |
| Routes | ? |
Mechanism of Action
Structure
- Aminocaproic acid is soluble in water, acid, and alkaline solutions; it is sparingly soluble in methanol and practically insoluble in chloroform.
- aminocaproic acid oral solution for oral administration, contains 0.25 g/mL of aminocaproic acid with methylparaben 0.20%, propylparaben 0.05%, edetate disodium 0.30% as preservatives and the following inactive ingredients: sodium saccharin, sorbitol solution, citric acid anhydrous, natural and artificial raspberry flavor and an artificial bitterness modifier.
- Each aminocaproic acid (aminocaproic acid) Tablet, for oral administration contains 500 mg or 1000 mg of aminocaproic acid and the following inactive ingredients: povidone, crospovidone, stearic acid, and magnesium stearate.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Aminocaproic acid (oral) in the drug label.
Pharmacokinetics
- The fibrinolysis-inhibitory effects of aminocaproic acid appear to be exerted principally via inhibition of plasminogen activators and to a lesser degree through antiplasmin activity.
- In adults, oral absorption appears to be a zero-order process with an absorption rate of 5.2 g/hr. The mean lag time in absorption is 10 minutes. After a single oral dose of 5 g, absorption was complete (F=1). Mean ± SD peak plasma concentrations (164 ± 28 mcg/mL) were reached within 1.2 ± 0.45 hours.
- After oral administration, the apparent volume of distribution was estimated to be 23.1 ± 6.6 L (mean ± SD). Correspondingly, the volume of distribution after intravenous administration has been reported to be 30.0 ± 8.2 L. After prolonged administration, aminocaproic acid has been found to distribute throughout extravascular and intravascular compartments of the body, penetrating human red blood cells as well as other tissue cells.
- Renal excretion is the primary route of elimination. Sixty-five percent of the dose is recovered in the urine as unchanged drug and 11% of the dose appears as the metabolite adipic acid. Renal clearance (116 mL/min) approximates endogenous creatinine clearance. The total body clearance is 169 mL/min. The terminal elimination half-life for aminocaproic acid is approximately 2 hours.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Aminocaproic acid (oral) in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Aminocaproic acid (oral) in the drug label.
How Supplied
- Aminocaproic acid oral solution, 0.25 g/mL
- Each mL of raspberry-flavored oral solution contains 0.25 g/mL of aminocaproic acid.
- 16 Fl. Oz. (473 mL) Bottle – NDC 49411-052-16
- AMICAR 500 mg Tablets
- Each round, white tablet, engraved with XP on one side and scored on the other with A to the left of the score and 10 on the right, contains 500 mg of aminocaproic acid.
- Bottle of 100 – NDC 49411-050-01
- AMICAR 1000 mg Tablets
- Each oblong, white tablet, engraved with XP on one side and scored on the other with A to the left of the score and 20 on the right, contains 1000 mg of aminocaproic acid.
- Bottle of 100 – NDC 49411-051-01
Storage
- Store at 20°-25°C (68°-77°F); Dispense in Tight Containers; Do Not Freeze.
Images
Drug Images
{{#ask: Page Name::Aminocaproic acid (oral) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel




{{#ask: Label Page::Aminocaproic acid (oral) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Aminocaproic acid (oral) in the drug label.
Precautions with Alcohol
- Alcohol-Aminocaproic acid (oral) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- AMICAR®[1]
Look-Alike Drug Names
There is limited information regarding Aminocaproic acid (oral) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=Aminocaproic acid (oral) |Pill Name=Aminocaproic_Acid_NDC_617480045.jpg |Drug Name=Aminocaproic Acid |Pill Ingred=AMINOCAPROIC ACID[AMINOCAPROIC ACID]|+sep=; |Pill Imprint=VP;045 |Pill Dosage=500 mg |Pill Color=White|+sep=; |Pill Shape=Round |Pill Size (mm)=13 |Pill Scoring=2 |Pill Image= |Drug Author=VersaPharm Incorporated |NDC=617480045
}}
{{#subobject:
|Page Name=Aminocaproic acid (oral) |Pill Name=Amicar_NDC_664790021.jpg |Drug Name=Amicar |Pill Ingred=Aminocaproic Acid[Aminocaproic Acid]|+sep=; |Pill Imprint=XP;A;10 |Pill Dosage=500 mg |Pill Color=White|+sep=; |Pill Shape=Round |Pill Size (mm)=13 |Pill Scoring=2 |Pill Image= |Drug Author=Xanodyne Pharmaceuticals, Inc. |NDC=664790021
}}
