Ambrisentan: Difference between revisions
No edit summary |
No edit summary |
||
| Line 99: | Line 99: | ||
* Pregnancy | * Pregnancy | ||
:* Letairis may cause fetal harm when administered to a pregnant female. Letairis is contraindicated in females who are pregnant. Letairis was consistently shown to have teratogenic effects when administered to animals. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to a fetus | :* Letairis may cause fetal harm when administered to a pregnant female. Letairis is contraindicated in females who are pregnant. Letairis was consistently shown to have teratogenic effects when administered to animals. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to a fetus. | ||
* Idiopathic Pulmonary Fibrosis | * Idiopathic Pulmonary Fibrosis | ||
:* Letairis is contraindicated in patients with Idiopathic Pulmonary Fibrosis (IPF), including IPF patients with pulmonary hypertension (WHO Group 3) | :* Letairis is contraindicated in patients with Idiopathic Pulmonary Fibrosis (IPF), including IPF patients with pulmonary hypertension (WHO Group 3) | ||
| Line 112: | Line 112: | ||
======Letairis REMS Program====== | ======Letairis REMS Program====== | ||
* For all females, Letairis is available only through a restricted program called the Letairis REMS, because of the risk of embryo-fetal toxicity | * For all females, Letairis is available only through a restricted program called the Letairis REMS, because of the risk of embryo-fetal toxicity. | ||
* Notable requirements of the Letairis REMS program include the following: | * Notable requirements of the Letairis REMS program include the following: | ||
:* Prescribers must be certified with the program by enrolling and completing training. | :* Prescribers must be certified with the program by enrolling and completing training. | ||
:* All females, regardless of reproductive potential, must enroll in the Letairis REMS program prior to initiating Letairis. Male patients are not enrolled in the REMS. | :* All females, regardless of reproductive potential, must enroll in the Letairis REMS program prior to initiating Letairis. Male patients are not enrolled in the REMS. | ||
::* Females of reproductive potential must comply with the pregnancy testing and contraception requirements | ::* Females of reproductive potential must comply with the pregnancy testing and contraception requirements. | ||
:* Pharmacies that dispense Letairis must be certified with the program and must dispense to female patients who are authorized to receive Letairis. | :* Pharmacies that dispense Letairis must be certified with the program and must dispense to female patients who are authorized to receive Letairis. | ||
* Further information is available at www.letairisrems.com or 1-866-664-5327. | * Further information is available at www.letairisrems.com or 1-866-664-5327. | ||
======Fluid Retention====== | ======Fluid Retention====== | ||
* Peripheral edema is a known class effect of endothelin receptor antagonists, and is also a clinical consequence of PAH and worsening PAH. In the placebo-controlled studies, there was an increased incidence of peripheral edema in patients treated with doses of 5 or 10 mg Letairis compared to placebo | * Peripheral edema is a known class effect of endothelin receptor antagonists, and is also a clinical consequence of PAH and worsening PAH. In the placebo-controlled studies, there was an increased incidence of peripheral edema in patients treated with doses of 5 or 10 mg Letairis compared to placebo. Most edema was mild to moderate in severity, and it occurred with greater frequency and severity in elderly patients. | ||
In addition, there have been postmarketing reports of fluid retention in patients with pulmonary hypertension, occurring within weeks after starting Letairis. Patients required intervention with a diuretic, fluid management, or, in some cases, hospitalization for decompensating heart failure. | * In addition, there have been postmarketing reports of fluid retention in patients with pulmonary hypertension, occurring within weeks after starting Letairis. Patients required intervention with a diuretic, fluid management, or, in some cases, hospitalization for decompensating heart failure. | ||
* If clinically significant fluid retention develops, with or without associated weight gain, further evaluation should be undertaken to determine the cause, such as Letairis or underlying heart failure, and the possible need for specific treatment or discontinuation of Letairis therapy. | * If clinically significant fluid retention develops, with or without associated weight gain, further evaluation should be undertaken to determine the cause, such as Letairis or underlying heart failure, and the possible need for specific treatment or discontinuation of Letairis therapy. | ||
Revision as of 13:45, 27 July 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gerald Chi
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: EMBRYO-FETAL TOXICITY
See full prescribing information for complete Boxed Warning.
* Do not administer Letairis to a pregnant female because it may cause fetal harm. Letairis is very likely to produce serious birth defects if used by pregnant females, as this effect has been seen consistently when it is administered to animals.
|
Overview
Ambrisentan is an endothelin receptor antagonist that is FDA approved for the {{{indicationType}}} of pulmonary arterial hypertension. There is a Black Box Warning for this drug as shown here. Common adverse reactions include edema, decreased hemoglobin, and headache.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Pulmonary Arterial Hypertension
- Letairis is indicated for the treatment of pulmonary arterial hypertension (PAH) (WHO Group 1) to improve exercise ability and delay clinical worsening. Studies establishing effectiveness included predominantly patients with WHO Functional Class II–III symptoms and etiologies of idiopathic or heritable PAH (64%) or PAH associated with connective tissue diseases (32%).
- Dosing Information
- Initiate treatment at 5 mg once daily, and consider increasing the dose to 10 mg once daily if 5 mg is tolerated.
- Tablets may be administered with or without food. Tablets should not be split, crushed, or chewed. Doses higher than 10 mg once daily have not been studied in patients with pulmonary arterial hypertension (PAH).
Pregnancy Testing in Females of Reproductive Potential
- Initiate treatment with Letairis in females of reproductive potential only after a negative pregnancy test. Obtain monthly pregnancy tests during treatment.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Ambrisentan in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Ambrisentan in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- Safety and effectiveness of Letairis in pediatric patients have not been established.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Ambrisentan in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Ambrisentan in pediatric patients.
Contraindications
- Pregnancy
- Letairis may cause fetal harm when administered to a pregnant female. Letairis is contraindicated in females who are pregnant. Letairis was consistently shown to have teratogenic effects when administered to animals. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to a fetus.
- Idiopathic Pulmonary Fibrosis
- Letairis is contraindicated in patients with Idiopathic Pulmonary Fibrosis (IPF), including IPF patients with pulmonary hypertension (WHO Group 3)
Warnings
|
WARNING: EMBRYO-FETAL TOXICITY
See full prescribing information for complete Boxed Warning.
* Do not administer Letairis to a pregnant female because it may cause fetal harm. Letairis is very likely to produce serious birth defects if used by pregnant females, as this effect has been seen consistently when it is administered to animals.
|
Embryo-fetal Toxicity
- Letairis may cause fetal harm when administered during pregnancy and is contraindicated for use in females who are pregnant. In females of reproductive potential, exclude pregnancy prior to initiation of therapy, ensure use of acceptable contraceptive methods, and obtain monthly pregnancy tests.
- Letairis is only available for females through a restricted program under a REMS.
Letairis REMS Program
- For all females, Letairis is available only through a restricted program called the Letairis REMS, because of the risk of embryo-fetal toxicity.
- Notable requirements of the Letairis REMS program include the following:
- Prescribers must be certified with the program by enrolling and completing training.
- All females, regardless of reproductive potential, must enroll in the Letairis REMS program prior to initiating Letairis. Male patients are not enrolled in the REMS.
- Females of reproductive potential must comply with the pregnancy testing and contraception requirements.
- Pharmacies that dispense Letairis must be certified with the program and must dispense to female patients who are authorized to receive Letairis.
- Further information is available at www.letairisrems.com or 1-866-664-5327.
Fluid Retention
- Peripheral edema is a known class effect of endothelin receptor antagonists, and is also a clinical consequence of PAH and worsening PAH. In the placebo-controlled studies, there was an increased incidence of peripheral edema in patients treated with doses of 5 or 10 mg Letairis compared to placebo. Most edema was mild to moderate in severity, and it occurred with greater frequency and severity in elderly patients.
- In addition, there have been postmarketing reports of fluid retention in patients with pulmonary hypertension, occurring within weeks after starting Letairis. Patients required intervention with a diuretic, fluid management, or, in some cases, hospitalization for decompensating heart failure.
- If clinically significant fluid retention develops, with or without associated weight gain, further evaluation should be undertaken to determine the cause, such as Letairis or underlying heart failure, and the possible need for specific treatment or discontinuation of Letairis therapy.
Pulmonary Edema with Pulmonary Veno-occlusive Disease (PVOD)
- If patients develop acute pulmonary edema during initiation of therapy with vasodilating agents such as Letairis, the possibility of PVOD should be considered, and if confirmed Letairis should be discontinued.
Decreased Sperm Counts
- Decreased sperm counts have been observed in human and animal studies with another endothelin receptor antagonist and in animal fertility studies with ambrisentan. Letairis may have an adverse effect on spermatogenesis. Counsel patients about potential effects on fertility.
Hematological Changes
- Decreases in hemoglobin concentration and hematocrit have followed administration of other endothelin receptor antagonists and were observed in clinical studies with Letairis. These decreases were observed within the first few weeks of treatment with Letairis, and stabilized thereafter. The mean decrease in hemoglobin from baseline to end of treatment for those patients receiving Letairis in the 12-week placebo-controlled studies was 0.8 g/dL.
- Marked decreases in hemoglobin (>15% decrease from baseline resulting in a value below the lower limit of normal) were observed in 7% of all patients receiving Letairis (and 10% of patients receiving 10 mg) compared to 4% of patients receiving placebo. The cause of the decrease in hemoglobin is unknown, but it does not appear to result from hemorrhage or hemolysis.
- In the long-term open-label extension of the two pivotal clinical studies, mean decreases from baseline (ranging from 0.9 to 1.2 g/dL) in hemoglobin concentrations persisted for up to 4 years of treatment.
- There have been postmarketing reports of decreases in hemoglobin concentration and hematocrit that have resulted in anemia requiring transfusion.
- Measure hemoglobin prior to initiation of Letairis, at one month, and periodically thereafter. Initiation of Letairis therapy is not recommended for patients with clinically significant anemia. If a clinically significant decrease in hemoglobin is observed and other causes have been excluded, consider discontinuing Letairis.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Clinical Trial Experience of Ambrisentan in the drug label.
Body as a Whole
Cardiovascular
Digestive
Endocrine
Hematologic and Lymphatic
Metabolic and Nutritional
Musculoskeletal
Neurologic
Respiratory
Skin and Hypersensitivy Reactions
Special Senses
Urogenital
Miscellaneous
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Ambrisentan in the drug label.
Body as a Whole
Cardiovascular
Digestive
Endocrine
Hematologic and Lymphatic
Metabolic and Nutritional
Musculoskeletal
Neurologic
Respiratory
Skin and Hypersensitivy Reactions
Special Senses
Urogenital
Miscellaneous
Drug Interactions
- Drug
- Description
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Ambrisentan in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Ambrisentan during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Ambrisentan with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Ambrisentan with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Ambrisentan with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Ambrisentan with respect to specific gender populations.
Race
There is no FDA guidance on the use of Ambrisentan with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Ambrisentan in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Ambrisentan in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Ambrisentan in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Ambrisentan in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
- Intravenous
Monitoring
There is limited information regarding Monitoring of Ambrisentan in the drug label.
- Description
IV Compatibility
There is limited information regarding IV Compatibility of Ambrisentan in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- Description
Management
- xx
Chronic Overdose
There is limited information regarding Chronic Overdose of Ambrisentan in the drug label.
Pharmacology
| |
Ambrisentan
| |
| Systematic (IUPAC) name | |
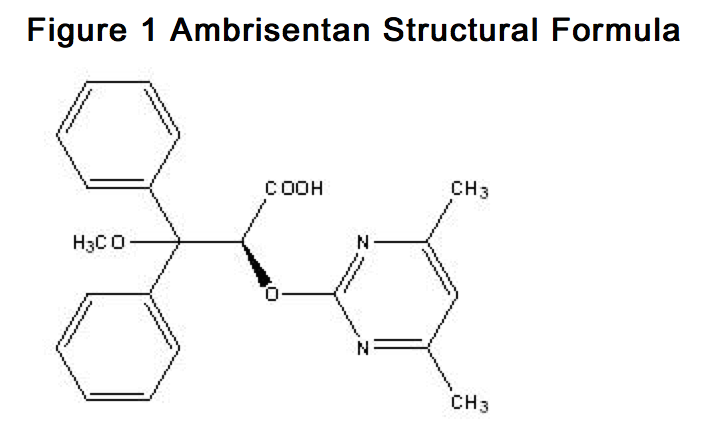
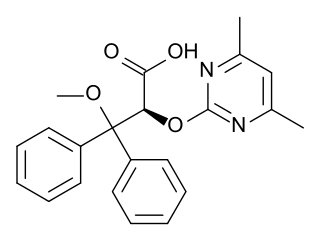
| (2S)-2-[(4,6-dimethylpyrimidin-2-yl)oxy]-3-methoxy- 3,3-diphenylpropanoic acid | |
| Identifiers | |
| CAS number | |
| ATC code | C02 |
| PubChem | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 378.421 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | Undetermined |
| Protein binding | 99% |
| Metabolism | ? |
| Half life | 15 hours (terminal) |
| Excretion | ? |
| Therapeutic considerations | |
| Licence data |
, |
| Pregnancy cat. | |
| Legal status |
Prescription Only (S4)(AU) ?(CA) POM(UK) [[Prescription drug|Template:Unicode-only]](US) |
| Routes | Oral |
Mechanism of Action
Structure
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Ambrisentan in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Ambrisentan in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Ambrisentan in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Ambrisentan in the drug label.
How Supplied
Storage
There is limited information regarding Ambrisentan Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Ambrisentan |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Ambrisentan |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Ambrisentan in the drug label.
Precautions with Alcohol
- Alcohol-Ambrisentan interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Letairis®[1]
Look-Alike Drug Names
- Letairis® — Letaris®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "LETAIRIS (ambrisentan) tablet, film coated".
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Ambrisentan |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Ambrisentan |Label Name=Ambrisentan11.png
}}
{{#subobject:
|Label Page=Ambrisentan |Label Name=Ambrisentan11.png
}}