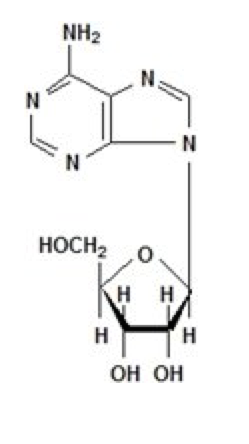
Adenosine
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gerald Chi
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Adenosine is an adenosine receptor agonist and antiarrhythmic that is FDA approved for the {{{indicationType}}} of paroxysmal supraventricular tachycardia (PSVT), including that associated with accessory bypass tracts (Wolff-Parkinson-White syndrome).. Common adverse reactions include chest discomfort, flushing, abdominal discomfort, pain of head and neck region, dizziness, headache, and dyspnea.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Paroxysmal Supraventricular Tachycardia
- Adenocard (adenosine injection) should be given as a rapid bolus by the peripheral intravenous route. To be certain the solution reaches the systemic circulation, it should be administered either directly into a vein or, if given into an IV line, it should be given as close to the patient as possible and followed by a rapid saline flush.
Adult Patients
- The dose recommendation is based on clinical studies with peripheral venous bolus dosing. Central venous (CVP or other) administration of Adenocard has not been systematically studied.
- Dosing Information
- Initial dose: 6 mg given as a rapid intravenous bolus (administered over a 1-2 second period).
- Repeat administration: If the first dose does not result in elimination of the supraventricular tachycardia within 1-2 minutes, 12 mg should be given as a rapid intravenous bolus. This 12 mg dose may be repeated a second time if required.
Pediatric Patients with a Body Weight < 50 kg
- Dosing Information
- Give 0.05 to 0.1 mg/kg as a rapid IV bolus given either centrally or peripherally. A saline flush should follow.
- Repeat administration: If conversion of PSVT does not occur within 1-2 minutes, additional bolus injections of adenosine can be administered at incrementally higher doses, increasing the amount given by 0.05 to 0.1 mg/kg. Follow each bolus with a saline flush. This process should continue until sinus rhythm is established or a maximum single dose of 0.3 mg/kg is used.
Pediatric Patients with a Body Weight ≥ 50 kg
- Administer the adult dose.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Supraventricular Tachycardia
- Developed by: ACCF/AHA
- Class of Recommendation: Class I
- Strength of Evidence: Category B
- Dosing Information
- 6 mg, followed by 12 mg as needed[1]
Non–Guideline-Supported Use
Preventing Graft Occlusion Following Aortocoronary Bypass Surgery
- Dosing Information
Percutaneous Transluminal Angioplasty
- Dosing Information
- 20 mg in 50 milliliters (mL) saline infused at a rate of 2 mg/minute[5]
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Adenosine in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Supraventricular Tachycardia
- Developed by: ACCF/AHA
- Class of Recommendation: Class IIa
- Strength of Evidence: Category B
- Dosing Information
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Adenosine in pediatric patients.
Contraindications
- Second- or third-degree A-V block (except in patients with a functioning artificial pacemaker)
- Sinus node disease, such as sick sinus syndrome or symptomatic bradycardia (except in patients with a functioning artificial pacemaker)
- Hypersensitivity to adenosine
Warnings
- Description
Precautions
- Description
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Clinical Trial Experience of Adenosine in the drug label.
Body as a Whole
Cardiovascular
Digestive
Endocrine
Hematologic and Lymphatic
Metabolic and Nutritional
Musculoskeletal
Neurologic
Respiratory
Skin and Hypersensitivy Reactions
Special Senses
Urogenital
Miscellaneous
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Adenosine in the drug label.
Body as a Whole
Cardiovascular
Digestive
Endocrine
Hematologic and Lymphatic
Metabolic and Nutritional
Musculoskeletal
Neurologic
Respiratory
Skin and Hypersensitivy Reactions
Special Senses
Urogenital
Miscellaneous
Drug Interactions
- Drug
- Description
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Adenosine in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Adenosine during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Adenosine with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Adenosine with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Adenosine with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Adenosine with respect to specific gender populations.
Race
There is no FDA guidance on the use of Adenosine with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Adenosine in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Adenosine in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Adenosine in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Adenosine in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
- Intravenous
Monitoring
There is limited information regarding Monitoring of Adenosine in the drug label.
Condition1
- Description
IV Compatibility
There is limited information regarding IV Compatibility of Adenosine in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- Description
Management
- Description
Chronic Overdose
There is limited information regarding Chronic Overdose of Adenosine in the drug label.
Pharmacology
There is limited information regarding Adenosine Pharmacology in the drug label.
Mechanism of Action
Structure
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Adenosine in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Adenosine in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Adenosine in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Adenosine in the drug label.
Condition1
- Description
How Supplied
Storage
There is limited information regarding Adenosine Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Adenosine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Adenosine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Adenosine in the drug label.
Precautions with Alcohol
- Alcohol-Adenosine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Adenocard®[10]
Look-Alike Drug Names
- A® — B®[11]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ DiMarco, J. P. (1990-07-15). "Adenosine for paroxysmal supraventricular tachycardia: dose ranging and comparison with verapamil. Assessment in placebo-controlled, multicenter trials. The Adenosine for PSVT Study Group". Annals of Internal Medicine. 113 (2): 104–110. ISSN 0003-4819. PMID 2193560. Unknown parameter
|coauthors=ignored (help) - ↑ Robinson, M. C. (1997-06). "Transient ventricular asystole using adenosine during minimally invasive and open sternotomy coronary artery bypass grafting". The Annals of Thoracic Surgery. 63 (6 Suppl): –30-34. ISSN 0003-4975. PMID 9203593. Unknown parameter
|coauthors=ignored (help); Check date values in:|date=(help) - ↑ Mentzer, R. M. (1997-06-19). "Safety, tolerance, and efficacy of adenosine as an additive to blood cardioplegia in humans during coronary artery bypass surgery". The American Journal of Cardiology. 79 (12A): 38–43. ISSN 0002-9149. PMID 9223362. Unknown parameter
|coauthors=ignored (help) - ↑ Mentzer, R. M. (1999-05). "Adenosine myocardial protection: preliminary results of a phase II clinical trial". Annals of Surgery. 229 (5): 643–649, discussion 649-650. ISSN 0003-4932. PMC 1420808. PMID 10235522. Unknown parameter
|coauthors=ignored (help); Check date values in:|date=(help) - ↑ Leesar, M. A. (1997-06-03). "Preconditioning of human myocardium with adenosine during coronary angioplasty". Circulation. 95 (11): 2500–2507. ISSN 0009-7322. PMID 9184580. Unknown parameter
|coauthors=ignored (help) - ↑ Losek, J. D. (1999-02). "Adenosine and pediatric supraventricular tachycardia in the emergency department: multicenter study and review". Annals of Emergency Medicine. 33 (2): 185–191. ISSN 0196-0644. PMID 9922414. Unknown parameter
|coauthors=ignored (help); Check date values in:|date=(help) - ↑ Lenk, M. (1997-10). "Role of adenosine in the diagnosis and treatment of tachyarrhythmias in pediatric patients". Acta Paediatrica Japonica; Overseas Edition. 39 (5): 570–577. ISSN 0374-5600. PMID 9363655. Unknown parameter
|coauthors=ignored (help); Check date values in:|date=(help) - ↑ Paret, G. (1996-08). "Adenosine for the treatment of paroxysmal supraventricular tachycardia in full-term and preterm newborn infants". American Journal of Perinatology. 13 (6): 343–346. doi:10.1055/s-2007-994353. ISSN 0735-1631. PMID 8865979. Unknown parameter
|coauthors=ignored (help); Check date values in:|date=(help) - ↑ Till, J. (1989-09). "Efficacy and safety of adenosine in the treatment of supraventricular tachycardia in infants and children". British Heart Journal. 62 (3): 204–211. ISSN 0007-0769. PMC 1216763. PMID 2789912. Unknown parameter
|coauthors=ignored (help); Check date values in:|date=(help) - ↑ "ADENOCARD (adenosine) solution".
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Adenosine |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Adenosine |Label Name=Adenosine11.png
}}
{{#subobject:
|Label Page=Adenosine |Label Name=Adenosine11.png
}}