Acyclovir (oral): Difference between revisions
Gerald Chi- (talk | contribs) No edit summary |
m (Protected "Acyclovir (oral)": Bot: Protecting all pages from category Drug ([Edit=Allow only administrators] (indefinite) [Move=Allow only administrators] (indefinite))) |
||
| (2 intermediate revisions by one other user not shown) | |||
| Line 565: | Line 565: | ||
}} | }} | ||
{{PillImage | {{PillImage | ||
|fileName= | |fileName=Acyclovir_NDC_605055306.jpg | ||
|drugName=acyclovir | |drugName=acyclovir | ||
|NDC=605055306 | |NDC=605055306 | ||
| Line 663: | Line 663: | ||
}} | }} | ||
{{PillImage | {{PillImage | ||
|fileName= | |fileName=Acyclovir_NDC_605055307.jpg | ||
|drugName=acyclovir | |drugName=acyclovir | ||
|NDC=605055307 | |NDC=605055307 | ||
Latest revision as of 17:13, 18 August 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Deepika Beereddy, MBBS [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Acyclovir (oral) is a antiviral agent that is FDA approved for the treatment of herpes zoster, genital herpes, and chickenpox. Common adverse reactions include nausea, vomiting, diarrhea, and malaise.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Herpes Zoster Infections
- Acyclovir is indicated for the acute treatment of herpes zoster (shingles).
- Dosing Information
- 800 mg every 4 hours orally, 5 times daily for 7 to 10 days.
- Chronic Suppressive Therapy for Recurrent Disease: 400 mg 2 times daily for up to 12 months, followed by re-evaluation. Alternative regimens have included doses ranging from 200 mg 3 times daily to 200 mg 5 times daily.
- The frequency and severity of episodes of untreated genital herpes may change over time. After 1 year of therapy, the frequency and severity of the patient’s genital herpes infection should be re-evaluated to assess the need for continuation of therapy with acyclovir.
- Intermittent Therapy: 200 mg every 4 hours, 5 times daily for 5 days. Therapy should be initiated at the earliest sign or symptom (prodrome) of recurrence.
Genital Herpes
- Acyclovir is indicated for the treatment of initial episodes and the management of recurrent episodes of genital herpes.
- Dosing Information
- 800 mg every 4 hours orally, 5 times daily for 7 to 10 days for treatment of initial genital herpes.
Chickenpox
- Acyclovir is indicated for the treatment of chickenpox (varicella).
- Children (2 Years of age and Older): 20 mg/kg per dose orally 4 times daily (80 mg/kg/day) for 5 days. Children over 40 kg should receive the adult dose for chickenpox.
- Adults and Children Over 40 kg: 800 mg 4 times daily for 5 days.
- Intravenous acyclovir is indicated for the treatment of varicella-zoster infections in immunocompromised patients.
- When therapy is indicated, it should be initiated at the earliest sign or symptom of chickenpox. There is no information about the efficacy of therapy initiated more than 24 hours after onset of signs and symptoms.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Acyclovir in adult patients.
Non–Guideline-Supported Use
Genital herpes simplex - HIV infection
- Dosing information:
- (oral) initial episode, 400 mg ORALLY 3 times daily for 7 to 10 days or 200 mg ORALLY 5 times daily for 10 days.
Herpes labialis - HIV infection
- Dosing information:
- 400 mg ORALLY 3 times daily for 5 to 10 days
Herpes zoster, Shingles - HIV infection
- Dosing informatioin:
- 800 mg ORALLY 5 times daily for 7 to 10 days
HIV infection - Varicella
- Dosing information:
- (uncomplicated cases) 20 mg/kg (MAX, 800 mg) ORALLY 5 times daily for 5 to 7 days
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Recurrent herpes simplex labialis
- Dosing information:
- For the treatment of recurrent herpes labialis (cold sores) in children 12 years or older, topical acyclovir 5% cream (Zovirax(R) Cream) should be applied 5 times daily for 4 days; therapy should be initiated as soon as possible after onset of signs/symptoms.
Varicella
- Dosing information:
- In children 2 years or older and weighing 40 kg or less, administer 20 mg/kg 4 times daily (80 mg/kg/day) for 5 days. In children over 40 kg, administer the adult dose of 800 mg 4 times daily for 5 days.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Acyclovir in pediatric patients.
Non–Guideline-Supported Use
Genital herpes simplex=
- Dosing Information:
- Initial Episode: The recommended dose for an initial genital herpes episode in children 12 years or older is 1000 to 1200 mg/day orally in 3 to 5 divided doses for 7 to 10 days
- Recurrent Episode: The recommended dose for recurrence of genital herpes in children 12 years or older is 1000 to 1200 mg/day orally in 3 divided doses for 3 to 5 days.
- Suppressive Therapy: The recommended dose for suppressive therapy for recurrent genital herpes in children 12 years or older is 800 to 1200 mg/day orally in 2 divided doses for up to 12 months.
Genital herpes simplex - HIV infection
- Dosing Information:
- The recommended dose of acyclovir for the treatment of genital herpes in children with HIV who weigh less than 45 kg is 20 mg/kg (maximum, 400 mg/dose) orally 3 times daily for 5 to 14 days. For adolescents, the dose of acyclovir is 400 mg orally twice daily for 5 to 14 days.
Herpes labialis - HIV infection
- Dosing Information:
- The recommended dose of acyclovir for the treatment of mild symptomatic gingivostomatitis due to herpes simplex virus in children with HIV is 20 mg/kg (maximum, 400 mg/dose) orally 3 times daily for 5 to 10 days
HIV infection- Varicella
- Dosing information:
- The recommended dose of acyclovir for the treatment of mild cases of chickenpox in children with HIV, and no or moderate immune suppression (CDC immunologic categories 1 and 2), is 20 mg/kg (maximum, 800 mg) orally 4 times daily for 7 to 10 days or until no new lesions appear for 48 hours.
Contraindications
- Acyclovir is contraindicated for patients who develop hypersensitivity to acyclovir or valacyclovir.
Warnings
- Acyclovir capsules and tablets are intended for oral ingestion only. Renal failure, in some cases resulting in death, has been observed with acyclovir therapy. Thrombotic thrombocytopenic purpura/hemolytic uremic syndrome (TTP/HUS), which has resulted in death, has occurred in immunocompromised patients receiving acyclovir therapy.
PRECAUTIONS
- Dosage adjustment is recommended when administering acyclovir to patients with renal impairment. Caution should also be exercised when administering acyclovir to patients receiving potentially nephrotoxic agents since this may increase the risk of renal dysfunction and/or the risk of reversible central nervous system symptoms such as those that have been reported in patients treated with intravenous acyclovir. Adequate hydration should be maintained.
Information for Patients
- Patients are instructed to consult with their physician if they experience severe or troublesome adverse reactions, they become pregnant or intend to become pregnant, they intend to breastfeed while taking orally administered acyclovir, or they have any other questions. Patients should be advised to maintain adequate hydration.
Herpes Zoster
- There are no data on treatment initiated more than 72 hours after onset of the zoster rash. Patients should be advised to initiate treatment as soon as possible after a diagnosis of herpes zoster.
Genital Herpes Infections
- Patients should be informed that acyclovir is not a cure for genital herpes. There are no data evaluating whether acyclovir will prevent transmission of infection to others. Because genital herpes is a sexually transmitted disease, patients should avoid contact with lesions or intercourse when lesions and/or symptoms are present to avoid infecting partners. Genital herpes can also be transmitted in the absence of symptoms through asymptomatic viral shedding. If medical management of a genital herpes recurrence is indicated, patients should be advised to initiate therapy at the first sign or symptom of an episode.
Chickenpox
- Chickenpox in otherwise healthy children is usually a self-limited disease of mild to moderate severity. Adolescents and adults tend to have more severe disease. Treatment was initiated within 24 hours of the typical chickenpox rash in the controlled studies, and there is no information regarding the effects of treatment begun later in the disease course.
Adverse Reactions
Clinical Trials Experience
Herpes Simplex
Short-Term Administration:
- The most frequent adverse events reported during clinical trials of treatment of genital herpes with acyclovir 200 mg administered orally 5 times daily every 4 hours for 10 days were nausea and/or vomiting in 8 of 298 patient treatments (2.7%). nausea and/or vomiting occurred in 2 of 287 (0.7%) patients who received placebo.
Long-Term Administration:
- The most frequent adverse events reported in a clinical trial for the prevention of recurrences with continuous administration of 400 mg (two 200 mg capsules) 2 times daily for 1 year in 586 patients treated with acyclovir were nausea (4.8%) and diarrhea (2.4%). The 589 control patients receiving intermittent treatment of recurrences with acyclovir for 1 year reported diarrhea (2.7%), nausea (2.4%), and headache (2.2%).
Herpes Zoster
- The most frequent adverse event reported during 3 clinical trials of treatment of herpes zoster (shingles) with 800 mg of oral acyclovir 5 times daily for 7 to 10 days in 323 patients was malaise (11.5%). The 323 placebo recipients reported malaise (11.1%).
Chickenpox
- The most frequent adverse event reported during 3 clinical trials of treatment of chickenpox with oral acyclovir at doses of 10 to 20 mg/kg 4 times daily for 5 to 7 days or 800 mg 4 times daily for 5 days in 495 patients was diarrhea (3.2%). The 498 patients receiving placebo reported diarrhea (2.2%).
Observed During Clinical Practice
- In addition to adverse events reported from clinical trials, the following events have been identified during post-approval use of acyclovir. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These events have been chosen for inclusion due to either their seriousness, frequency of reporting, potential causal connection to acyclovir, or a combination of these factors.
General
- Anaphylaxis, angioedema, fever, headache, pain, peripheral edema.
Nervous
- Aggressive behavior, agitation, ataxia, coma, confusion, decreased consciousness, delirium, dizziness, dysarthria, encephalopathy, hallucinations, paresthesia, psychosis, seizure, somnolence, tremors. These symptoms may be marked, particularly in older adults or in patients with renal impairment (see PRECAUTIONS).
Digestive
- Diarrhea, gastrointestinal distress, nausea.
Hematologic and Lymphatic
Hepatobiliary Tract and Pancreas
- Elevated liver function tests, hepatitis, hyperbilirubinemia, jaundice.
Musculoskeletal
Skin
- Alopecia, erythema multiforme, photosensitive rash, pruritus, rash, Stevens-Johnson syndrome, toxic epidermal necrolysis, urticaria.
Special Senses
- Visual abnormalities.
Urogenital
- Renal failure, renal pain (may be associated with renal failure), elevated blood urea nitrogen, elevated creatinine, hematuria.
Postmarketing Experience
Carcinogenesis, Mutagenesis, Impairment of Fertility
- The data presented below include references to peak steady-state plasma acyclovir concentrations observed in humans treated with 800 mg given orally 5 times a day (dosing appropriate for treatment of herpes zoster) or 200 mg given orally 5 times a day (dosing appropriate for treatment of genital herpes). Plasma drug concentrations in animal studies are expressed as multiples of human exposure to acyclovir at the higher and lower dosing schedules (see CLINICAL PHARMACOLOGY, Pharmacokinetics).
- Acyclovir was tested in lifetime bioassays in rats and mice at single daily doses of up to 450 mg/kg administered by gavage. There was no statistically significant difference in the incidence of tumors between treated and control animals, nor did acyclovir shorten the latency of tumors. Maximum plasma concentrations were 3 to 6 times human levels in the mouse bioassay and 1 to 2 times human levels in the rat bioassay.
- Acyclovir was tested in 16 in vitro and in vivo genetic toxicity assays. Acyclovir was positive in 5 of the assays.
- Acyclovir did not impair fertility or reproduction in mice (450 mg/kg/day, p.o.) or in rats (25 mg/kg/day, s.c.). In the mouse study, plasma levels were 9 to 18 times human levels, while in the rat study, they were 8 to 15 times human levels. At higher doses (50 mg/kg/day, s.c.) in rats and rabbits (11 to 22 and 16 to 31 times human levels, respectively) implantation efficacy, but not litter size, was decreased. In a rat peri- and post-natal study at 50 mg/kg/day, s.c., there was a statistically significant decrease in group mean numbers of corpora lutea, total implantation sites, and live fetuses.
- No testicular abnormalities were seen in dogs given 50 mg/kg/day, IV for 1 month (21 to 41 times human levels) or in dogs given 60 mg/kg/day orally for 1 year (6 to 12 times human levels). Testicular atrophy and aspermatogenesis were observed in rats and dogs at higher dose levels.
Drug Interactions
Coadministration of probenecid with acyclovir has been shown to increase the mean acyclovir half-life and the area under the concentration-time curve. Urinary excretion and renal clearance were correspondingly reduced.
Use in Specific Populations
Pregnancy
- Acyclovir administered during organogenesis was not teratogenic in the mouse (450 mg/kg/day, p.o.), rabbit (50 mg/kg/day, s.c. and IV), or rat (50 mg/kg/day, s.c.). These exposures resulted in plasma levels 9 and 18, 16 and 106, and 11 and 22 times, respectively, human levels.
- There are no adequate and well-controlled studies in pregnant women. A prospective epidemiologic registry of acyclovir use during pregnancy was established in 1984 and completed in April 1999. There were 749 pregnancies followed in women exposed to systemic acyclovir during the first trimester of pregnancy resulting in 756 outcomes. The occurrence rate of birth defects approximates that found in the general population. However, the small size of the registry is insufficient to evaluate the risk for less common defects or to permit reliable or definitive conclusions regarding the safety of acyclovir in pregnant women and their developing fetuses. Acyclovir should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Acyclovir (oral) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Acyclovir (oral) during labor and delivery.
Nursing Mothers
- Acyclovir concentrations have been documented in breast milk in 2 women following oral administration of acyclovir and ranged from 0.6 to 4.1 times corresponding plasma levels. These concentrations would potentially expose the nursing infant to a dose of acyclovir up to 0.3 mg/kg/day. Acyclovir should be administered to a nursing mother with caution and only when indicated.
Pediatric Use
- Safety and effectiveness of oral formulations of acyclovir in pediatric patients younger than 2 years of age have not been established.
Geriatic Use
- Of 376 subjects who received acyclovir in a clinical study of herpes zoster treatment in immunocompetent subjects ≥ 50 years of age, 244 were 65 and over while 111 were 75 and over. No overall differences in effectiveness for time to cessation of new lesion formation or time to healing were reported between geriatric subjects and younger adult subjects. The duration of pain after healing was longer in patients 65 and over. nausea, vomiting, and dizziness were reported more frequently in elderly subjects. Elderly patients are more likely to have reduced renal function and require dose reduction. Elderly patients are also more likely to have renal or CNS adverse events. With respect to CNS adverse events observed during clinical practice, somnolence, hallucinations, confusion, and coma were reported more frequently in elderly patients
Gender
There is no FDA guidance on the use of Acyclovir (oral) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Acyclovir (oral) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Acyclovir (oral) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Acyclovir (oral) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Acyclovir (oral) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Acyclovir (oral) in patients who are immunocompromised.
Others
Administration and Monitoring
Administration
Acute Treatment of Herpes Zoster
- 800 mg every 4 hours orally, 5 times daily for 7 to 10 days.
Genital Herpes
Treatment of Initial Genital Herpes
- 200 mg every 4 hours, 5 times daily for 10 days.
Chronic Suppressive Therapy for Recurrent Disease
- 400 mg 2 times daily for up to 12 months, followed by re-evaluation. Alternative regimens have included doses ranging from 200 mg 3 times daily to 200 mg 5 times daily.
- The frequency and severity of episodes of untreated genital herpes may change over time. After 1 year of therapy, the frequency and severity of the patient’s genital herpes infection should be re-evaluated to assess the need for continuation of therapy with acyclovir.
Intermittent Therapy
- 200 mg every 4 hours, 5 times daily for 5 days. Therapy should be initiated at the earliest sign or symptom (prodrome) of recurrence.
Treatment of Chickenpox
- Children (2 Years of age and Older): 20 mg/kg per dose orally 4 times daily (80 mg/kg/day) for 5 days. Children over 40 kg should receive the adult dose for chickenpox.
- Adults and Children Over 40 kg: 800 mg 4 times daily for 5 days.
- Intravenous acyclovir is indicated for the treatment of varicella-zoster infections in immunocompromised patients.
- When therapy is indicated, it should be initiated at the earliest sign or symptom of chickenpox. There is no information about the efficacy of therapy initiated more than 24 hours after onset of signs and symptoms.
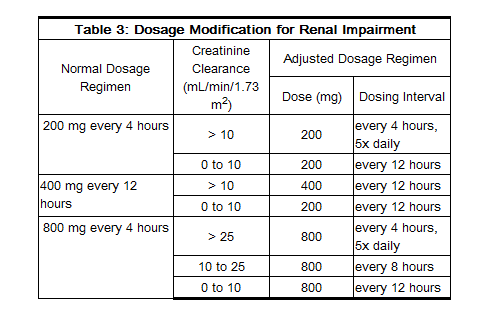
Patients With Acute or Chronic Renal Impairment
- In patients with renal impairment, the dose of acyclovir capsules and tablets should be modified as shown in Table 3:
Hemodialysis
- For patients who require hemodialysis, the mean plasma half-life of acyclovir during hemodialysis is approximately 5 hours. This results in a 60% decrease in plasma concentrations following a 6 hour dialysis period. Therefore, the patient’s dosing schedule should be adjusted so that an additional dose is administered after each dialysis.
Peritoneal Dialysis
- No supplemental dose appears to be necessary after adjustment of the dosing interval.
Bioequivalence of Dosage Forms
- Acyclovir suspension was shown to be bioequivalent to acyclovir capsules (n = 20) and 1 acyclovir 800 mg tablet was shown to be bioequivalent to 4 acyclovir 200 mg capsules (n = 24).
Monitoring
There is limited information regarding Acyclovir (oral) Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Acyclovir (oral) and IV administrations.
Overdosage
Overdoses involving ingestion of up to 100 capsules (20 g) have been reported. Adverse events that have been reported in association with overdosage include agitation, coma, seizures, and lethargy. Precipitation of acyclovir in renal tubules may occur when the solubility (2.5 mg/mL) is exceeded in the intratubular fluid. Overdosage has been reported following bolus injections or inappropriately high doses and in patients whose fluid and electrolyte balance were not properly monitored. This has resulted in elevated BUN and serum creatinine and subsequent renal failure. In the event of acute renal failure and anuria, the patient may benefit from hemodialysis until renal function is restored.
Pharmacology
Acyclovir (oral)
| |
| Systematic (IUPAC) name | |
| ? | |
| Identifiers | |
| CAS number | ? |
| ATC code | ? |
| PubChem | ? |
| Chemical data | |
| Formula | ? |
| Mol. mass | ? |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
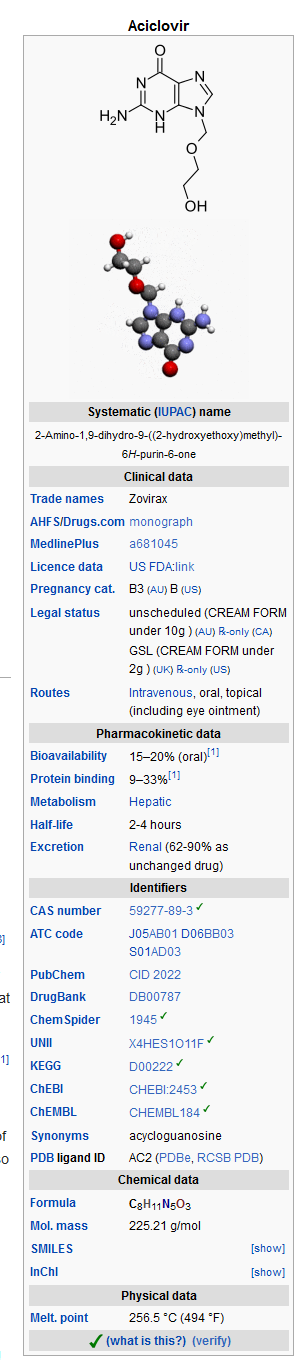
Mechanism of Action
Mechanism of Antiviral Action
- Acyclovir is a synthetic purine nucleoside analogue with in vitro and in vivo inhibitory activity against herpes simplex virus types 1 (HSV-1), 2 (HSV- 2), and varicella-zoster virus (VZV).
- The inhibitory activity of acyclovir is highly selective due to its affinity for the enzyme thymidine kinase (TK) encoded by HSV and VZV. This viral enzyme converts acyclovir into acyclovir monophosphate, a nucleotide analogue. The monophosphate is further converted into diphosphate by cellular guanylate kinase and into triphosphate by a number of cellular enzymes. In vitro, acyclovir triphosphate stops replication of herpes viral DNA. This is accomplished in 3 ways: 1) competitive inhibition of viral DNA polymerase, 2) incorporation into and termination of the growing viral DNA chain, and 3) inactivation of the viral DNA polymerase. The greater antiviral activity of acyclovir against HSV compared to VZV is due to its more efficient phosphorylation by the viral TK.
Antiviral Activities
- The quantitative relationship between the in vitro susceptibility of herpes viruses to antivirals and the clinical response to therapy has not been established in humans, and virus sensitivity testing has not been standardized. Sensitivity testing results, expressed as the concentration of drug required to inhibit by 50% the growth of virus in cell culture (IC50), vary greatly depending upon a number of factors. Using plaque-reduction assays, the IC50 against herpes simplex virus isolates ranges from 0.02 to 13.5 mcg/mL for HSV-1 and from 0.01 to 9.9 mcg/mL for HSV-2. The IC50 for acyclovir against most laboratory strains and clinical isolates of VZV ranges from 0.12 to 10.8 mcg/mL. Acyclovir also demonstrates activity against the Oka vaccine strain of VZV with a mean IC50 of 1.35 mcg/mL.
Drug Resistance
- Resistance of HSV and VZV to acyclovir can result from qualitative and quantitative changes in the viral TK and/or DNA polymerase. Clinical isolates of HSV and VZV with reduced susceptibility to acyclovir have been recovered from immunocompromised patients, especially with advanced HIV infection. While most of the acyclovir-resistant mutants isolated thus far from immunocompromised patients have been found to be TK-deficient mutants, other mutants involving the viral TK gene (TK partial and TK altered) and DNA polymerase have been isolated. TK-negative mutants may cause severe disease in infants and immunocompromised adults. The possibility of viral resistance to acyclovir should be considered in patients who show poor clinical response during therapy.
Structure
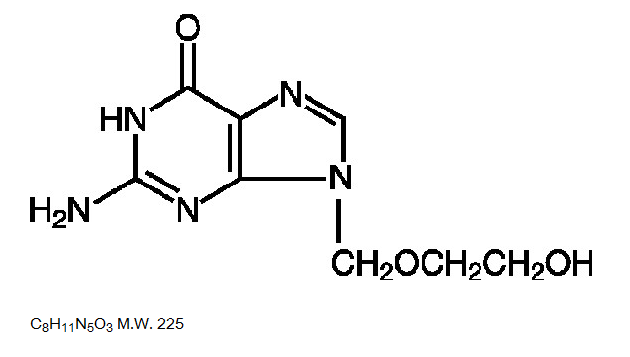
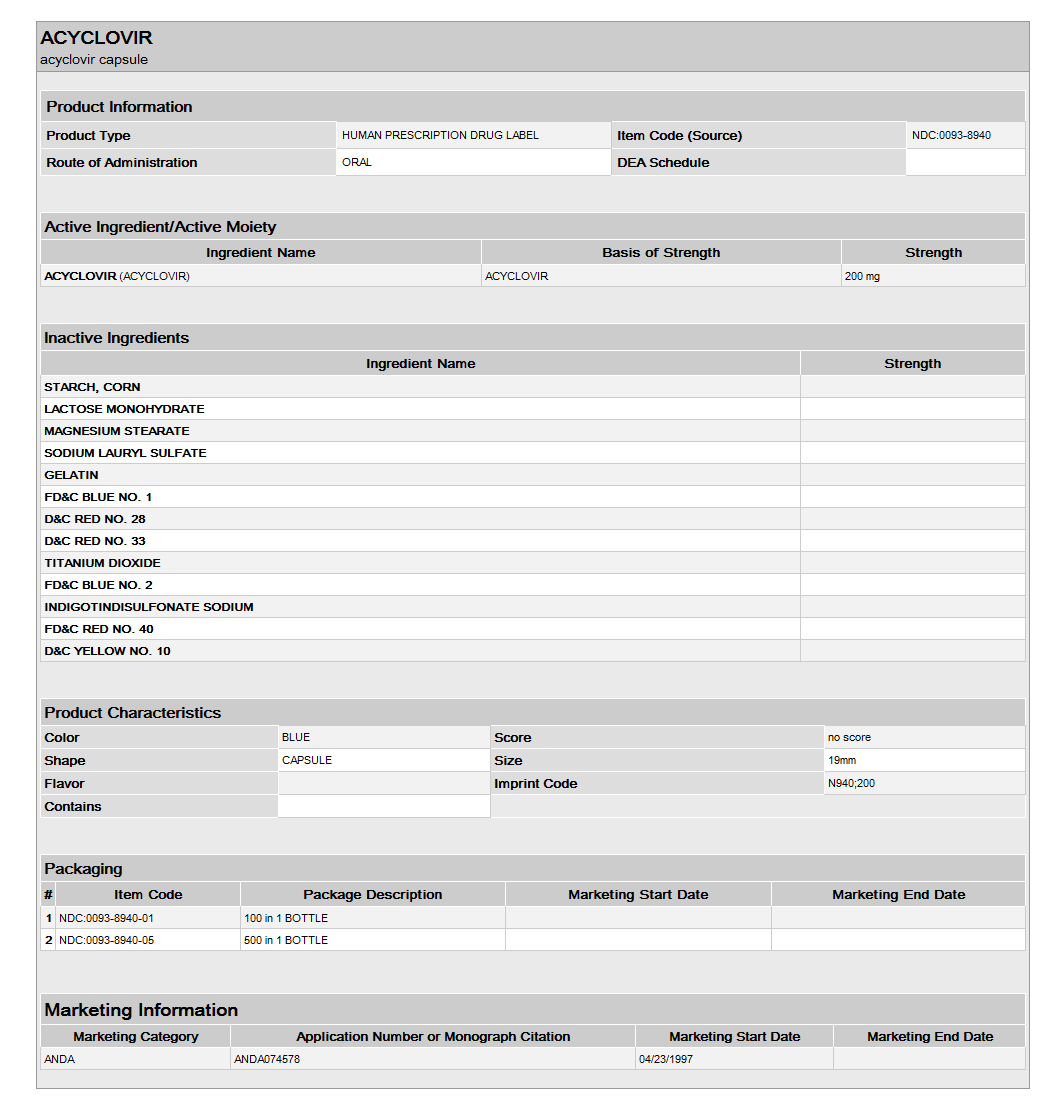
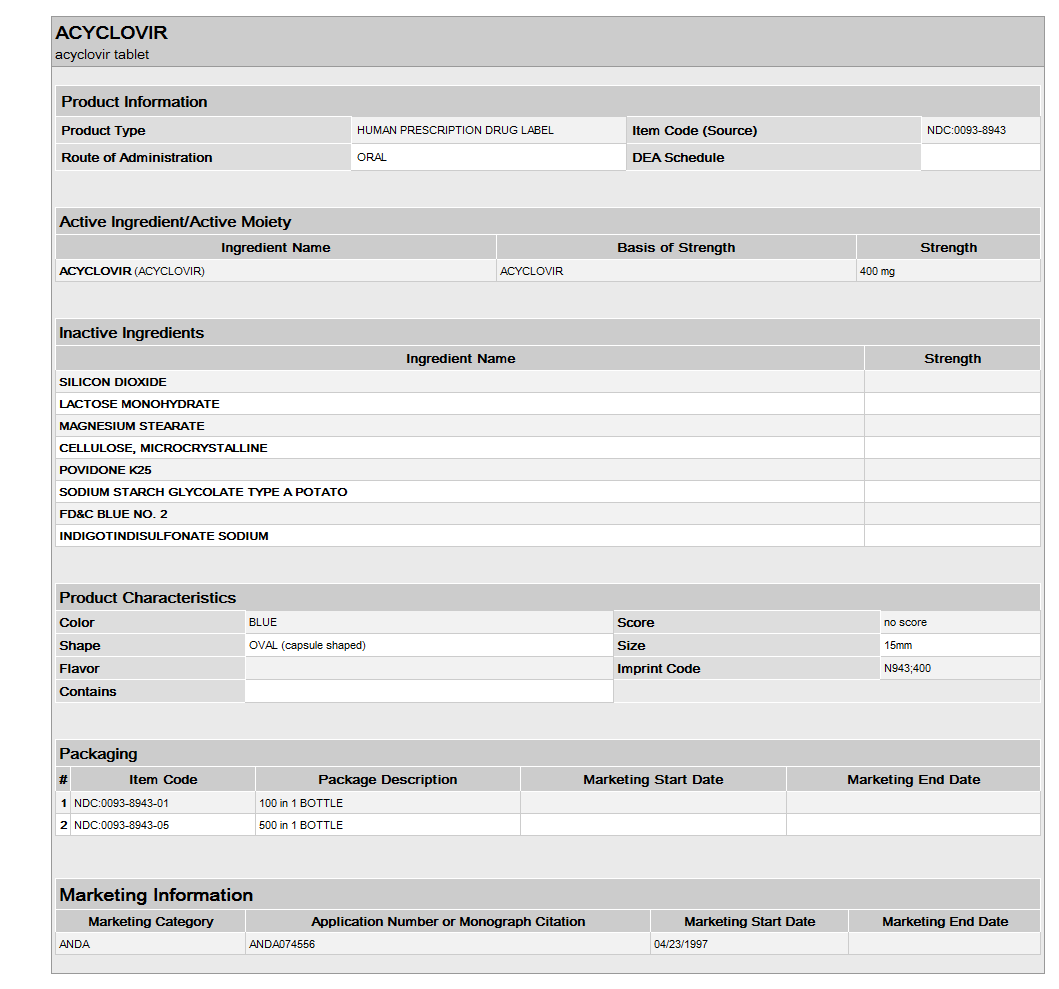
- Acyclovir is a synthetic nucleoside analogue active against herpesviruses. Each capsule, for oral administration, contains 200 mg of acyclovir. In addition, each capsule contains the following inactive ingredients: corn starch, lactose monohydrate, magnesium stearate and sodium lauryl sulfate. The capsule shell consists of gelatin, FD&C blue No. 1, D&C red No. 28, D&C red No. 33 and titanium dioxide. Printed with edible black ink that contains FD&C blue No. 1, FD&C blue No. 2, FD&C red No. 40 and D&C yellow No. 10. Each tablet, for oral administration, contains 400 mg or 800 mg of acyclovir. In addition, each tablet contains the following inactive ingredients: colloidal silicon dioxide, lactose monohydrate, magnesium stearate, microcrystalline cellulose, povidone, pregelatinized starch and sodium starch glycolate. The 400 mg tablets also contain FD&C blue No. 2.
- Acyclovir is a white to off-white, crystalline powder. The maximum solubility in water at 37°C is 2.5 mg/mL. The pka’s of acyclovir are 2.27 and 9.25.
- The chemical name of acyclovir is 2-amino-1,9-dihydro-9-[(2-hydroxyethoxy)methyl]-6H-purin-6-one; it has the following structural formula:
Pharmacodynamics
There is limited information regarding Acyclovir (oral) Pharmacodynamics in the drug label.
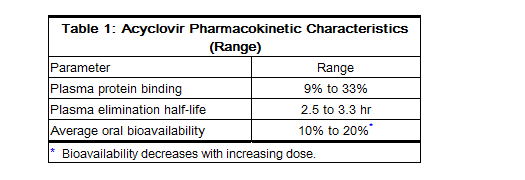
Pharmacokinetics
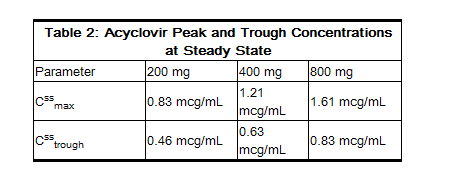
- The pharmacokinetics of acyclovir after oral administration have been evaluated in healthy volunteers and in immunocompromised patients with herpes simplex or varicella-zoster virus infection. Acyclovir pharmacokinetic parameters are summarized in Table 1.
- In one multiple-dose, crossover study in healthy subjects (n = 23), it was shown that increases in plasma acyclovir concentrations were less than dose proportional with increasing dose, as shown in Table 2. The decrease in bioavailability is a function of the dose and not the dosage form.
- There was no effect of food on the absorption of acyclovir (n = 6); therefore, acyclovir capsules and tablets may be administered with or without food.
- The only known urinary metabolite is 9-[(carboxymethoxy)methyl]guanine.
Special Populations
Adults With Impaired Renal Function
- The half-life and total body clearance of acyclovir are dependent on renal function. A dosage adjustment is recommended for patients with reduced renal function (see DOSAGE AND ADMINISTRATION).
Geriatrics
- Acyclovir plasma concentrations are higher in geriatric patients compared to younger adults, in part due to age-related changes in renal function. Dosage reduction may be required in geriatric patients with underlying renal impairment (see PRECAUTIONS, Geriatric Use).
Pediatrics
- In general, the pharmacokinetics of acyclovir in pediatric patients is similar to that of adults. Mean half-life after oral doses of 300 mg/m2 and 600 mg/m2 in pediatric patients aged 7 months to 7 years was 2.6 hours (range 1.59 to 3.74 hours).
Drug Interactions
- Coadministration of probenecid with intravenous acyclovir has been shown to increase the mean acyclovir half-life and the area under the concentration-time curve. Urinary excretion and renal clearance were correspondingly reduced.
Nonclinical Toxicology
There is limited information regarding Acyclovir (oral) Nonclinical Toxicology in the drug label.
Clinical Studies
Clinical Trials
Initial Genital Herpes
- Double-blind, placebo-controlled studies have demonstrated that orally administered acyclovir significantly reduced the duration of acute infection and duration of lesion healing. The duration of pain and new lesion formation was decreased in some patient groups.
Recurrent Genital Herpes
- Double-blind, placebo-controlled studies in patients with frequent recurrences (6 or more episodes per year) have shown that orally administered acyclovir given daily for 4 months to 10 years prevented or reduced the frequency and/or severity of recurrences in greater than 95% of patients.
- In a study of patients who received acyclovir 400 mg twice daily for 3 years, 45%, 52%, and 63% of patients remained free of recurrences in the first, second, and third years, respectively. Serial analyses of the 3 month recurrence rates for the patients showed that 71% to 87% were recurrence free in each quarter.
Herpes Zoster Infections
- In a double-blind, placebo-controlled study of immunocompetent patients with localized cutaneous zoster infection, acyclovir (800 mg 5 times daily for 10 days) shortened the times to lesion scabbing, healing, and complete cessation of pain, and reduced the duration of viral shedding and the duration of new lesion formation.
- In a similar double-blind, placebo-controlled study, acyclovir (800 mg 5 times daily for 7 days) shortened the times to complete lesion scabbing, healing, and cessation of pain; reduced the duration of new lesion formation; and reduced the prevalence of localized zoster-associated neurologic symptoms (paresthesia, dysesthesia, or hyperesthesia).
- Treatment was begun within 72 hours of rash onset and was most effective if started within the first 48 hours.
Adults greater than 50 years of age showed greater benefit.
Chickenpox
- Three randomized, double-blind, placebo-controlled trials were conducted in 993 pediatric patients aged 2 to 18 years with chickenpox. All patients were treated within 24 hours after the onset of rash. In 2 trials, acyclovir was administered at 20 mg/kg 4 times daily (up to 3,200 mg per day) for 5 days. In the third trial, doses of 10, 15, or 20 mg/kg were administered 4 times daily for 5 to 7 days. Treatment with acyclovir shortened the time to 50% healing; reduced the maximum number of lesions; reduced the median number of vesicles; decreased the median number of residual lesions on day 28; and decreased the proportion of patients with fever, anorexia, and lethargy by day 2. Treatment with acyclovir did not affect varicella-zoster virus-specific humoral or cellular immune responses at 1 month or 1 year following treatment.
How Supplied
Acyclovir capsules USP are available containing 200 mg acyclovir. Each opaque blue cap and body size #1 hard gelatin capsule is imprinted with black ink N 940 and 200 on opposing cap and body portion of the capsule.
They are supplied as follows:
NDC 0093-8940-01 Bottles of 100
NDC 0093-8940-05 Bottles of 500
NDC 0093-8940-93 Unit dose boxes of 100
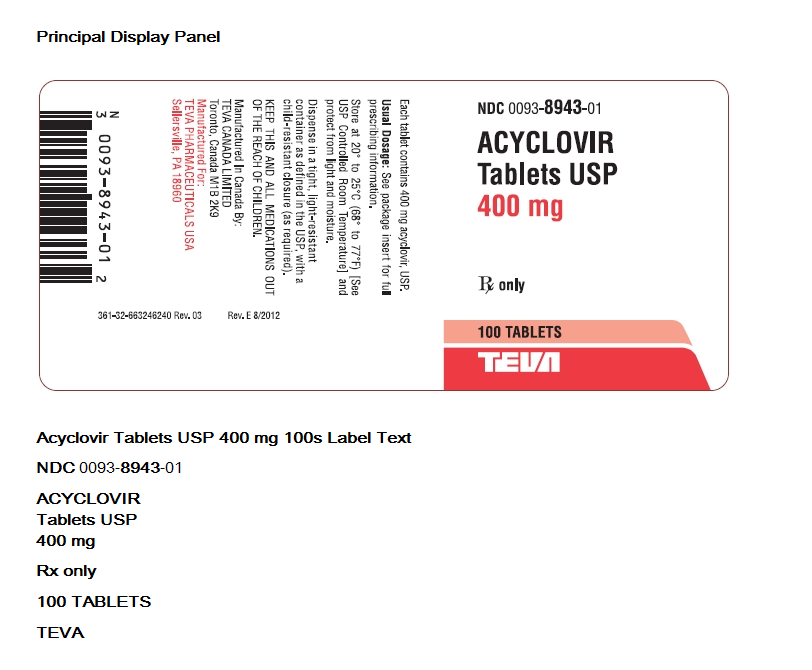
Acyclovir tablets USP are available containing 400 mg acyclovir. Each blue colored, biconvex, capsule shaped, compressed unscored tablet is debossed with N943 on one side and 400 on the other side.
They are supplied as follows:
NDC 0093-8943-01 Bottles of 100
NDC 0093-8943-05 Bottles of 500
NDC 0093-8943-93 Unit dose boxes of 100
Acyclovir tablets USP are available containing 800 mg acyclovir. Each white to off-white colored, biconvex, capsule shaped, compressed unscored tablet is debossed with N947 on one side and 800 on the other side.
They are supplied as follows:
NDC 0093-8947-01 Bottles of 100
NDC 0093-8947-05 Bottles of 500
NDC 0093-8947-93 Unit dose boxes of 100
Storage
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature]. Protect from light and moisture.
Images
Drug Images
{{#ask: Page Name::Acyclovir (oral) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel


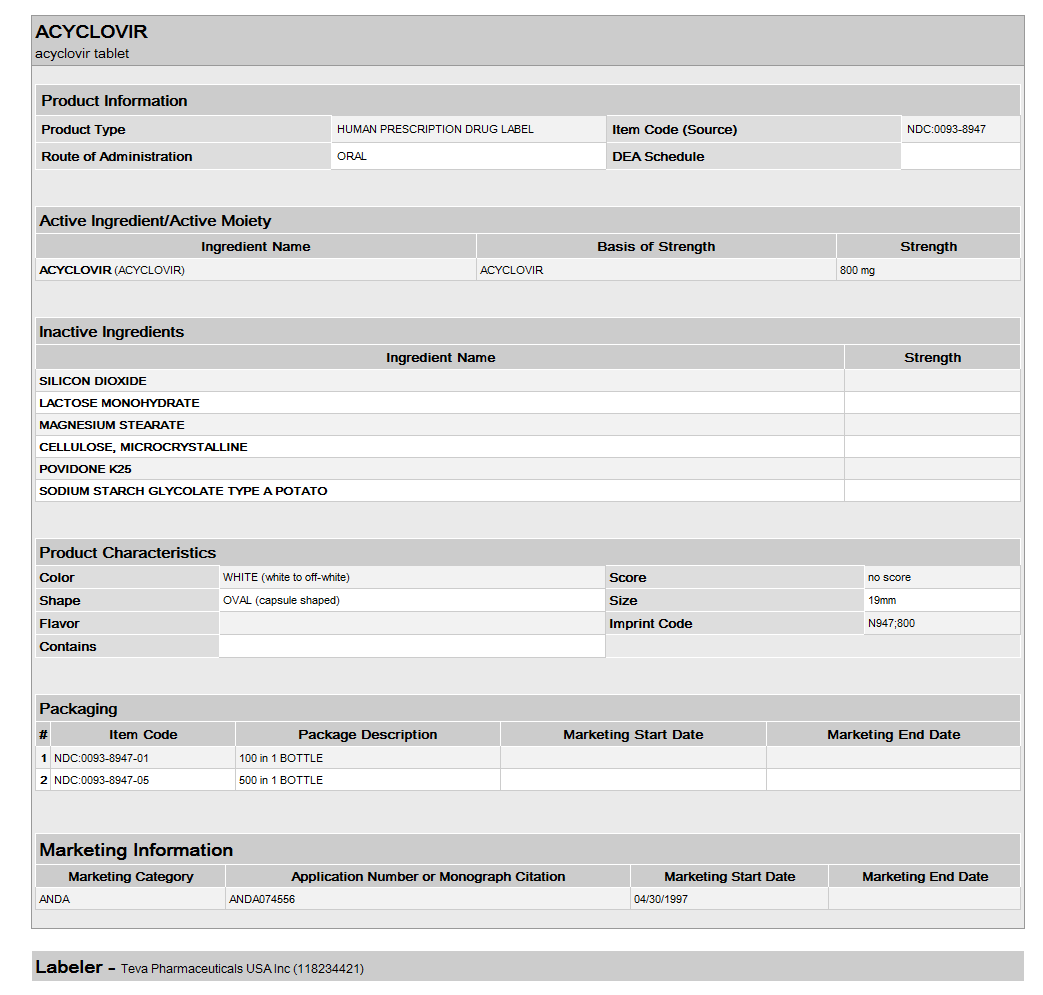
{{#ask: Label Page::Acyclovir (oral) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Patients are instructed to consult with their physician if they experience severe or troublesome adverse reactions, they become pregnant or intend to become pregnant, they intend to breastfeed while taking orally administered acyclovir, or they have any other questions. Patients should be advised to maintain adequate hydration.
Herpes Zoster
- There are no data on treatment initiated more than 72 hours after onset of the zoster rash. Patients should be advised to initiate treatment as soon as possible after a diagnosis of herpes zoster.
Genital Herpes Infections
- Patients should be informed that acyclovir is not a cure for genital herpes. There are no data evaluating whether acyclovir will prevent transmission of infection to others. Because genital herpes is a sexually transmitted disease, patients should avoid contact with lesions or intercourse when lesions and/or symptoms are present to avoid infecting partners. Genital herpes can also be transmitted in the absence of symptoms through asymptomatic viral shedding. If medical management of a genital herpes recurrence is indicated, patients should be advised to initiate therapy at the first sign or symptom of an episode.
Chickenpox
- Chickenpox in otherwise healthy children is usually a self-limited disease of mild to moderate severity. Adolescents and adults tend to have more severe disease. Treatment was initiated within 24 hours of the typical chickenpox rash in the controlled studies, and there is no information regarding the effects of treatment begun later in the disease course.
Precautions with Alcohol
Alcohol-Acyclovir interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Look-Alike Drug Names
There is limited information regarding Acyclovir (oral) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=Acyclovir_NDC_00938947.jpg |Drug Name=Acyclovir |Pill Ingred=ACYCLOVIR[ACYCLOVIR]|+sep=; |Pill Imprint=N947;800 |Pill Dosage=800 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=19 |Pill Scoring=1 |Pill Image= |Drug Author=Teva Pharmaceuticals USA Inc |NDC=00938947
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=Acyclovir_NDC_03780253.jpg |Drug Name=Acyclovir |Pill Ingred=ACYCLOVIR[ACYCLOVIR]|+sep=; |Pill Imprint=M;253 |Pill Dosage=400 mg |Pill Color=White|+sep=; |Pill Shape=Round |Pill Size (mm)=11 |Pill Scoring=1 |Pill Image= |Drug Author=Mylan Pharmaceuticals Inc. |NDC=03780253
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=Acyclovir_NDC_03780302.jpg |Drug Name=Acyclovir |Pill Ingred=Acyclovir[Acyclovir]|+sep=; |Pill Imprint=MYLAN;302 |Pill Dosage=800 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=19 |Pill Scoring=1 |Pill Image= |Drug Author=Mylan Pharmaceuticals Inc. |NDC=03780302
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=ACYCLOVIR_NDC_09045789.jpg |Drug Name=ACYCLOVIR |Pill Ingred=ACYCLOVIR[ACYCLOVIR]|+sep=; |Pill Imprint=APO;042 |Pill Dosage=200 mg |Pill Color=|+sep=; |Pill Shape=Capsule |Pill Size (mm)=18 |Pill Scoring=1 |Pill Image= |Drug Author=Major Pharmaceuticals |NDC=09045789
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=Acyclovir_NDC_422910108.jpg |Drug Name=Acyclovir |Pill Ingred=ACYCLOVIR[ACYCLOVIR]|+sep=; |Pill Imprint=CTI;112 |Pill Dosage=400 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=15 |Pill Scoring=1 |Pill Image= |Drug Author=AvKARE, Inc. |NDC=422910108
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=Acyclovir_NDC_422910109.jpg |Drug Name=Acyclovir |Pill Ingred=ACYCLOVIR[ACYCLOVIR]|+sep=; |Pill Imprint=CTI;113 |Pill Dosage=800 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=19 |Pill Scoring=1 |Pill Image= |Drug Author=AvKARE, Inc. |NDC=422910109
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=Acyclovir_NDC_604290711.jpg |Drug Name=Acyclovir |Pill Ingred=ACYCLOVIR[ACYCLOVIR]|+sep=; |Pill Imprint=200;A05 |Pill Dosage=200 mg |Pill Color=Blue|+sep=; |Pill Shape=Capsule |Pill Size (mm)=15 |Pill Scoring=1 |Pill Image= |Drug Author=Golden State Medical Supply, Inc. |NDC=604290711
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=Acyclovir_NDC_604290712.jpg |Drug Name=Acyclovir |Pill Ingred=ACYCLOVIR[ACYCLOVIR]|+sep=; |Pill Imprint=CTI;112 |Pill Dosage=400 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=15 |Pill Scoring=1 |Pill Image= |Drug Author=Golden State Medical Supply, Inc. |NDC=604290712
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=Acyclovir_NDC_604290713.jpg |Drug Name=Acyclovir |Pill Ingred=ACYCLOVIR[ACYCLOVIR]|+sep=; |Pill Imprint=CTI;113 |Pill Dosage=800 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=19 |Pill Scoring=1 |Pill Image= |Drug Author=Golden State Medical Supply, Inc. |NDC=604290713
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=Acyclovir_NDC_604290911.jpg |Drug Name=Acyclovir |Pill Ingred=ACYCLOVIR[ACYCLOVIR]|+sep=; |Pill Imprint=200;CTI;111; |Pill Dosage=200 mg |Pill Color=White|+sep=; |Pill Shape=Capsule |Pill Size (mm)=15 |Pill Scoring=1 |Pill Image= |Drug Author=Golden State Medical Supply, Inc. |NDC=604290911
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=Acyclovir_NDC_605055306.jpg |Drug Name=acyclovir |Pill Ingred=ACYCLOVIR[ACYCLOVIR]|+sep=; |Pill Imprint=Ax;5306 |Pill Dosage=400 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=15 |Pill Scoring=1 |Pill Image= |Drug Author=Apotex Corp. |NDC=605055306
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=Acyclovir_NDC_614420111.jpg |Drug Name=Acyclovir |Pill Ingred=Acyclovir[Acyclovir]|+sep=; |Pill Imprint=200;CTI;111 |Pill Dosage=200 mg |Pill Color=Blue|+sep=; |Pill Shape=Capsule |Pill Size (mm)=20 |Pill Scoring=1 |Pill Image= |Drug Author=Carlsbad Technology, Inc. |NDC=614420111
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=Acyclovir_NDC_633040505.jpg |Drug Name=Acyclovir |Pill Ingred=ACYCLOVIR[ACYCLOVIR]|+sep=; |Pill Imprint=RX505 |Pill Dosage=800 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=20 |Pill Scoring=1 |Pill Image= |Drug Author=Ranbaxy Laboratories Ltd. |NDC=633040505
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=Acyclovir_NDC_633040652.jpg |Drug Name=Acyclovir |Pill Ingred=ACYCLOVIR[ACYCLOVIR]|+sep=; |Pill Imprint=RX652 |Pill Dosage=200 mg |Pill Color=White|+sep=; |Pill Shape=Capsule |Pill Size (mm)=19 |Pill Scoring=1 |Pill Image= |Drug Author=Ranbaxy Laboratories Ltd. |NDC=633040652
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=Acyclovir_NDC_672530101.jpg |Drug Name=Acyclovir |Pill Ingred=ACYCLOVIR[ACYCLOVIR]|+sep=; |Pill Imprint=A02;400 |Pill Dosage=400 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=16 |Pill Scoring=1 |Pill Image= |Drug Author=DAVA Pharmaceuticals, Inc. |NDC=672530101
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=Acyclovir_NDC_00938940.jpg |Drug Name=Acyclovir |Pill Ingred=ACYCLOVIR[ACYCLOVIR]|+sep=; |Pill Imprint=N940;200 |Pill Dosage=200 mg |Pill Color=Blue|+sep=; |Pill Shape=Capsule |Pill Size (mm)=19 |Pill Scoring=1 |Pill Image= |Drug Author=Teva Pharmaceuticals USA Inc |NDC=00938940
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=Acyclovir_NDC_00938943.jpg |Drug Name=Acyclovir |Pill Ingred=ACYCLOVIR[ACYCLOVIR]|+sep=; |Pill Imprint=N943;400 |Pill Dosage=400 mg |Pill Color=Blue|+sep=; |Pill Shape=Oval |Pill Size (mm)=15 |Pill Scoring=1 |Pill Image= |Drug Author=Teva Pharmaceuticals USA Inc |NDC=00938943
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=Acyclovir_NDC_605055307.jpg |Drug Name=acyclovir |Pill Ingred=ACYCLOVIR[ACYCLOVIR]|+sep=; |Pill Imprint=Ax;5307 |Pill Dosage=800 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=19 |Pill Scoring=1 |Pill Image= |Drug Author=Apotex Corp. |NDC=605055307
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=Acyclovir_NDC_614420112.jpg |Drug Name=Acyclovir |Pill Ingred=Acyclovir[Acyclovir]|+sep=; |Pill Imprint=CTI;112 |Pill Dosage=400 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=15 |Pill Scoring=1 |Pill Image= |Drug Author=Carlsbad Technology, Inc. |NDC=614420112
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=Acyclovir_NDC_633040504.jpg |Drug Name=Acyclovir |Pill Ingred=ACYCLOVIR[ACYCLOVIR]|+sep=; |Pill Imprint=RX504 |Pill Dosage=400 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=14 |Pill Scoring=1 |Pill Image= |Drug Author=Ranbaxy Laboratories Ltd. |NDC=633040504
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=ZOVIRAX_NDC_01730945.jpg |Drug Name=ZOVIRAX |Pill Ingred=ACYCLOVIR[ACYCLOVIR]|+sep=; |Pill Imprint=ZOVIRAX;800 |Pill Dosage=800 mg |Pill Color=Blue|+sep=; |Pill Shape=Oval |Pill Size (mm)=19 |Pill Scoring=1 |Pill Image= |Drug Author=GlaxoSmithKline LLC |NDC=01730945
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=ZOVIRAX_NDC_01730949.jpg |Drug Name=ZOVIRAX |Pill Ingred=ACYCLOVIR[ACYCLOVIR]|+sep=; |Pill Imprint=ZOVIRAX |Pill Dosage=400 mg |Pill Color=White|+sep=; |Pill Shape=Hexagon |Pill Size (mm)=12 |Pill Scoring=1 |Pill Image= |Drug Author=GlaxoSmithKline LLC |NDC=01730949
}}
{{#subobject:
|Page Name=Acyclovir (oral) |Pill Name=ZOVIRAX_NDC_01730991.jpg |Drug Name=ZOVIRAX |Pill Ingred=ACYCLOVIR[ACYCLOVIR]|+sep=; |Pill Imprint=Wellcome;ZOVIRAX;200 |Pill Dosage=200 mg |Pill Color=Blue|+sep=; |Pill Shape=Capsule |Pill Size (mm)=20 |Pill Scoring=1 |Pill Image= |Drug Author=GlaxoSmithKline LLC |NDC=01730991
}}