21-hydroxylase deficiency pathophysiology
|
21-hydroxylase deficiency Microchapters |
|
Differentiating 21-Hydroxylase Deficiency from other Diseases |
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
21-hydroxylase deficiency pathophysiology On the Web |
|
American Roentgen Ray Society Images of 21-hydroxylase deficiency pathophysiology |
|
Risk calculators and risk factors for 21-hydroxylase deficiency pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor-In-Chief: Mehrian Jafarizade, M.D [2]
Overview
The progression to 21-hydroxylase deficiency usually involves the a defective conversion of 17-hydroxyprogesterone to 11-deoxycortisol which results in decreased cortisol synthesis and therefore increased corticotropin (ACTH) secretion. The resulting adrenal stimulation leads to increased production of androgens due to shunting of the pathway to androgen synthesis. More than 95% of cases of congenital adrenal hyperplasia (CAH) are caused by 21-hydroxylase deficiency. The clinical manifestations of congenital adrenal hyperplasia is closely related to the type and severity of disease. The severity of disease relates to the mutation type which is causes enzyme inactivity or hypo-activity. There is a lack of enzyme in classic form of 21-hydroxylase deficiency; while in the non-classic form, enzymatic activity is reduced but sufficient to maintain normal glucocorticoid and mineralocorticoid production. The gene responsible for 21-hydroxylase deficiency is CYP21A. This gene is located within the human leucocyte antigen class III region of chromosome 6. Meiotic recombination occurs in this genomic region as a result of the high degree of sequence homology between CYP21A2 and its pseudogene CYP21A1. Approximately 70% of CYP21A2 genetic mutation is due to gene conversion and micro-deletions in CYP21A1 gene.
Pathophysiology
- 21-hydroxylase enzyme is involved in the synthesis of aldosterone, in zona glumerulosa and cortisol in zona fasciculata. Lack of this enzyme leads to decrease in cortisol and aldosterone levels and the rest of synthesis pathways goes to produce extra androgens and leads to hirsutism.
- More than 95% of all cases of congenital adrenal hyperplasia (CAH) are caused by 21-hydroxylase deficiency; the clinical manifestations of 21-hydroxylase deficiency is closely related to the type and severity of disease.
- The severity of disease relates to the mutation type, which casues enzyme inactivity or hypo activity.
- There is a lack of enzyme in classic type of 21-hydroxylase deficiency; while in the non-classic form, enzymatic activity is reduced but sufficient to maintain normal glucocorticoid and mineralocorticoid production.
Glucocorticoid status
- In patients with 21-hydroxylase deficiency, in zona fasciculata, there is a defective conversion of 17-hydroxyprogesterone to 11-deoxycortisol which results in decreased cortisol synthesis and therefore increased corticotropin (ACTH) secretion.
Mineralocorticoids status
- In patients with 21-hydroxylase deficiency, in zona glomerolosa, there is a defective conversion of progesterone to 11-deoxycortisterone which results in decreased aldosterone synthesis.The lack of aldosterone causes large amounts of sodium loss in the urine. Urinary sodium concentrations are more than 50 mEq/L. As a result of high amount of sodium loss, blood volume and blood pressure can not be maintained in normal ranges. In mineralocorticoid deficiency setting, potassium and acid excretion are also impaired, result in hyperkalemia and metabolic acidosis.There is a significant water loss and dehydration symptoms due to salt wasting within the first two week of life. In severe CAH forms vomiting, severe dehydration, and circulatory collapse and shock will develop in the second or third week of life.
Androgen status
- In the androgen synthesis pathway, 21-hydroxylase enzyme has no role; therefore with extra amount of other products from blocked cortisol and aldosterone synthesis, androgen pathway have extra precursor metabolites; result in androgen excess in the form of dehydroepianrosterone and androstenedione accumulation. On the other hand lack of cortisol removes the negative feedback on the pituitary, result in increase in ACTH level and consequently more increase in androgen synthesis pathway. High androgen level in 21 hydroxylase deficient women during pregnancy causes ambiguous genitalia in female fetus; also in milder forms induces hirsutism and virilization in women. Adrenal androgens produce little effect on the genitalia of male infants with severe CAH. Exccess androgen can cause precocious puberty in male child.
Below is the hormonal pathway of adrenal steroids and related enzymes.[1][2]
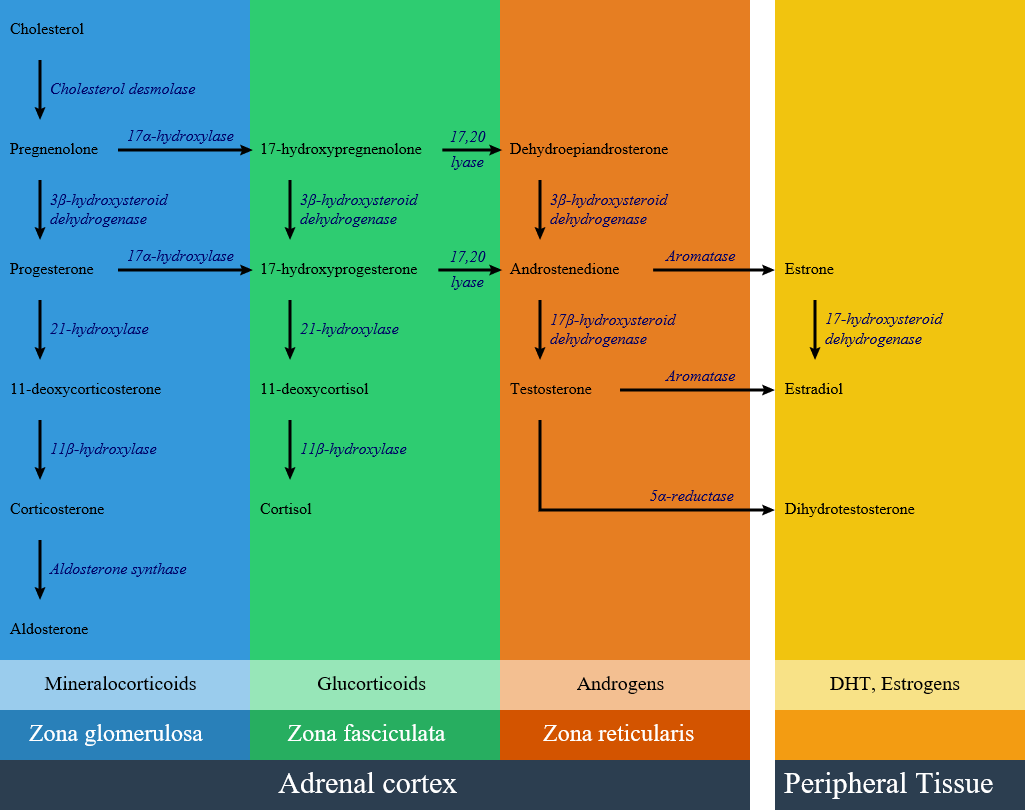
Genetics
- Congenital adrenal hyperplasia subtypes are all autosomal recessive and monogenetic. The disease manifestation follows the allele that results in a more functional enzyme, and generally correlation between genotype and phenotype is good.[4][5][4]
CYP21A gene
- The gene responsible for 21-hydroxylase deficiency is CYP21A. This gene is located within the human leucocyte antigen class III region of chromosome 6.
CYP21A gene has two types:
CYP21A2
- An active gene called CYP21A2, which encodes 21-hydroxylase, a cytochrome P450 type II enzyme of 495 amino acids.
CYP21A1
- This gene is a non-functional pseudogene named CYP21A1 or CYP21P. This pseudogene produces an enzyme with no activity because it lacks eight bases from codons 110-112, which results in a stop codon.[6]
Mutation mechanisms:
- Meiotic recombination events occurs in this genomic region as a result of the high degree of sequence homology between CYP21A2 and its pseudogene CYP21A1.
- Approximately 70% of CYP21A2 disease is due to gene conversion and microdeletions in CYP21A1 gene.
- Approximately 25% to 30% are chimeric genes due to large deletions.
- Approximately 1% to 2% of cases are due to de novo mutations because of high variability of the CYP21A2 locus.
- Chromosome 6 uniparental disomy is rare cause of 21-hydroxylase deficiency with an unknown prevalence.
- Gene mutations that completely inactivate CYP21A2 gene will result in the classic type and salt-wasting subtype.
- Gene mutations that maintain 1–2% of 21-hydroxylase activity will result in classic type and non-salt-wasting subtype. These patients have minimal aldosterone production that prevents a neonatal adrenal crisis.[7]
Gross Pathology
Gross pathology findings in patients with 21 hydroxylase deficiency are:[8][9]
- Enlarged adrenal glands
- Wrinkled surface of adrenal glands
- Cerebriform pattern in adrenal glands (pathognomonic sign)
- Normal ultrasound appearance
- Testicular masses may be identified representing adrenal rest tissue
Microscopic Pathology
In 21 hydroxylase deficiency microscopic findings may include:
- Diffuse cortical hyperplasia with smaller cells
- The cell cytoplasm can be vacuolated, and often more basophilic.
- Rare mitotic figures may be present
- The hyperplastic cells typically lack features of cellular atypia.[10]
 |
 |
References
- ↑ White PC, Speiser PW (2000). "Congenital adrenal hyperplasia due to 21-hydroxylase deficiency". Endocr. Rev. 21 (3): 245–91. doi:10.1210/edrv.21.3.0398. PMID 10857554.
- ↑ Speiser PW, Azziz R, Baskin LS, Ghizzoni L, Hensle TW, Merke DP, Meyer-Bahlburg HF, Miller WL, Montori VM, Oberfield SE, Ritzen M, White PC (2010). "Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society clinical practice guideline". J. Clin. Endocrinol. Metab. 95 (9): 4133–60. doi:10.1210/jc.2009-2631. PMC 2936060. PMID 20823466.
- ↑ "File:Adrenal Steroids Pathways.svg - Wikimedia Commons".
- ↑ 4.0 4.1 Finkielstain GP, Chen W, Mehta SP, Fujimura FK, Hanna RM, Van Ryzin C, McDonnell NB, Merke DP (2011). "Comprehensive genetic analysis of 182 unrelated families with congenital adrenal hyperplasia due to 21-hydroxylase deficiency". J. Clin. Endocrinol. Metab. 96 (1): E161–72. doi:10.1210/jc.2010-0319. PMC 3038490. PMID 20926536.
- ↑ New MI, Abraham M, Gonzalez B, Dumic M, Razzaghy-Azar M, Chitayat D, Sun L, Zaidi M, Wilson RC, Yuen T (2013). "Genotype-phenotype correlation in 1,507 families with congenital adrenal hyperplasia owing to 21-hydroxylase deficiency". Proc. Natl. Acad. Sci. U.S.A. 110 (7): 2611–6. doi:10.1073/pnas.1300057110. PMC 3574953. PMID 23359698.
- ↑ White PC, New MI, Dupont B (1986). "Structure of human steroid 21-hydroxylase genes". Proc. Natl. Acad. Sci. U.S.A. 83 (14): 5111–5. PMC 323900. PMID 3487786.
- ↑ Fiet J, Gueux B, Gourmelen M, Kuttenn F, Vexiau P, Couillin P, Pham-Huu-Trung MT, Villette JM, Raux-Demay MC, Galons H (1988). "Comparison of basal and adrenocorticotropin-stimulated plasma 21-deoxycortisol and 17-hydroxyprogesterone values as biological markers of late-onset adrenal hyperplasia". J. Clin. Endocrinol. Metab. 66 (4): 659–67. doi:10.1210/jcem-66-4-659. PMID 2831244.
- ↑ Congenital adrenal hyperplasia. Dr Henry Knipe and Dr M Venkatesh . Radiopaedia.org 2015.http://radiopaedia.org/articles/congenital-adrenal-hyperplasia
- ↑ Teixeira SR, Elias PC, Andrade MT, Melo AF, Elias Junior J (2014). "The role of imaging in congenital adrenal hyperplasia". Arq Bras Endocrinol Metabol. 58 (7): 701–8. PMID 25372578.
- ↑ 10.0 10.1 10.2 "Adrenal Gland - Hyperplasia - Nonneoplastic Lesion Atlas".