Oligodendroglioma pathophysiology
|
Oligodendroglioma Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Oligodendroglioma pathophysiology On the Web |
|
American Roentgen Ray Society Images of Oligodendroglioma pathophysiology |
|
Risk calculators and risk factors for Oligodendroglioma pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [4]Associate Editor(s)-in-Chief: Sara Mohsin, M.D.[5]Sujit Routray, M.D. [6]
Overview
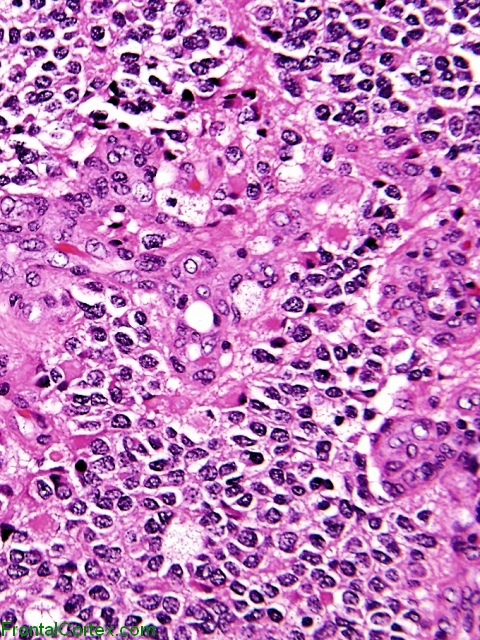
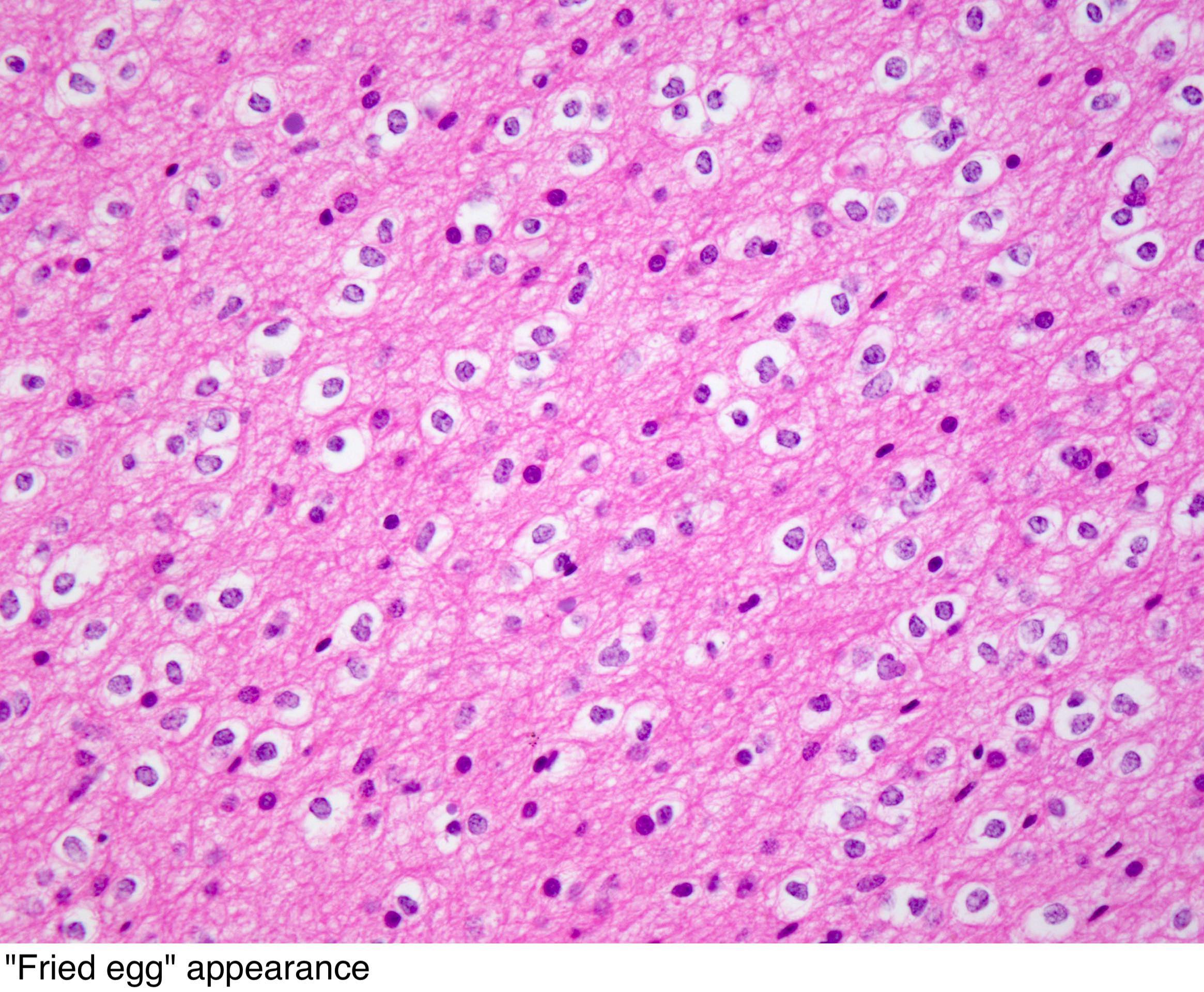
Oligodendroglioma arises from the tripotential glial precursor cells and not from the bipotential oligodendrocytes. Genes associated with the pathogenesis of oligodendroglioma include t[1;19][q10;p10], ATRX, NJDS, IDH1, IDH2, TERT promoter, H3 K27M (H3F3A, HIST1H3B/C), CIC, FUBP1, p53, Leu-7, TCF-12, TP53,MGMT, TP73, BRAF, EGFR, and PTEN. Common intracranial sites involved by oligodendroglioma include cerebral hemispheres, posterior fossa, and intramedullary spinal cord. On gross pathology, oligodendroglioma is characterized by a well-circumscribed, gelatinous, calcified, cystic, gray mass with focal hemorrhage which may expand a gyrus and remodel the skull. On microscopic histopathological analysis, oligodendroglioma is characterized by diffuse growthpattern of highly cellular lesion of monomorphic cells having rounded nucleus with atypia, speckled "salt-and-pepper" chromatin pattern and perinuclear haloresembling fried eggs, distinct cell borders, clear cytoplasm, abundant calcification and "chicken-wire" like vascularity pattern. Oligodendroglioma is demonstrated by positivity to tumor markers such as IDH1-R132H, MAP2, GFAP, S-100, SOX10, EMA, ATRX, Ki-67, NSE, synaptophysin, OLIG1, and OLIG2.
Pathophysiology
Pathogenesis
- Oligodendroglioma does not arise from the bipotential oligodendrocytes, although the tumor cells look very similiar[1]
- Oligodendroglioma arises from the tripotential glial precursor cells
Genetics
- Development of oligodendroglioma is the result of multiple genetic mutations[2][3][4][5][6][7][8][9][10][11][12]
- Genes associated with the pathogenesis of oligodendroglioma include:[13][14][15][16][17][18][19][20][21][22][23][24][25]
- t(1;19)(q10;p10) (co-deletion of chromosomal arms 1p36 and 19q13; most common)[26][10][27]
- ATRX[28]
- IDH1[29][30][31][32][33][34][35][36][37]
- IDH2[38][39][40][41][42][43]
- TERT promoter[22][44][45][46][47][48]
- H3 K27M mutations in either H3F3A (one of two genes encoding the histone H3.3 variant) or HIST1H3B/C (encoding the histone H3.1 variant)[49][50][51][52][53][54]
- NJDS (A 2009 Oxford Neurosymposium study illustrated that there's a 69% correlation between NJDS gene mutation and tumor initiation)
- CIC[55][16]
- FUBP1
- TP53
- p53[56]
- BRAF alterations:[57]
- Leu-7
- TCF-12
- MGMT
- TP73
- EGFR
- PTEN
- There is a strong association of oligodendroglioma with expression of receptor tyrosine kinases that activate PI3K/AKT, RAS/MAP, and PLC/PKC pathways[20]
Gross Pathology
- On gross pathology, oligodendroglioma is characterized by a well-circumscribed, gelatinous, gray mass which may expand a gyrus and remodel the skull[58]
- Other characteristic gross pathological features associated with oligodendroglioma include:[58][20]
- Calcification (70-90%; one of the most frequently calcifying tumors)
- Focal hemorrhage
- Cystic (20%)
- Common intracranial sites associated with oligodendroglioma include:[59]
- Cerebral hemispheres (cortex and white matter) - distribution between frontal (most common, > 50% of cases), parietal, temporal, and occipital lobe approximates 3:2:2:1
- Posterior fossa (rare)
- Intramedullary spinal cord (very rare, only 1.5% of oligodendrogliomas)
Microscopic Pathology
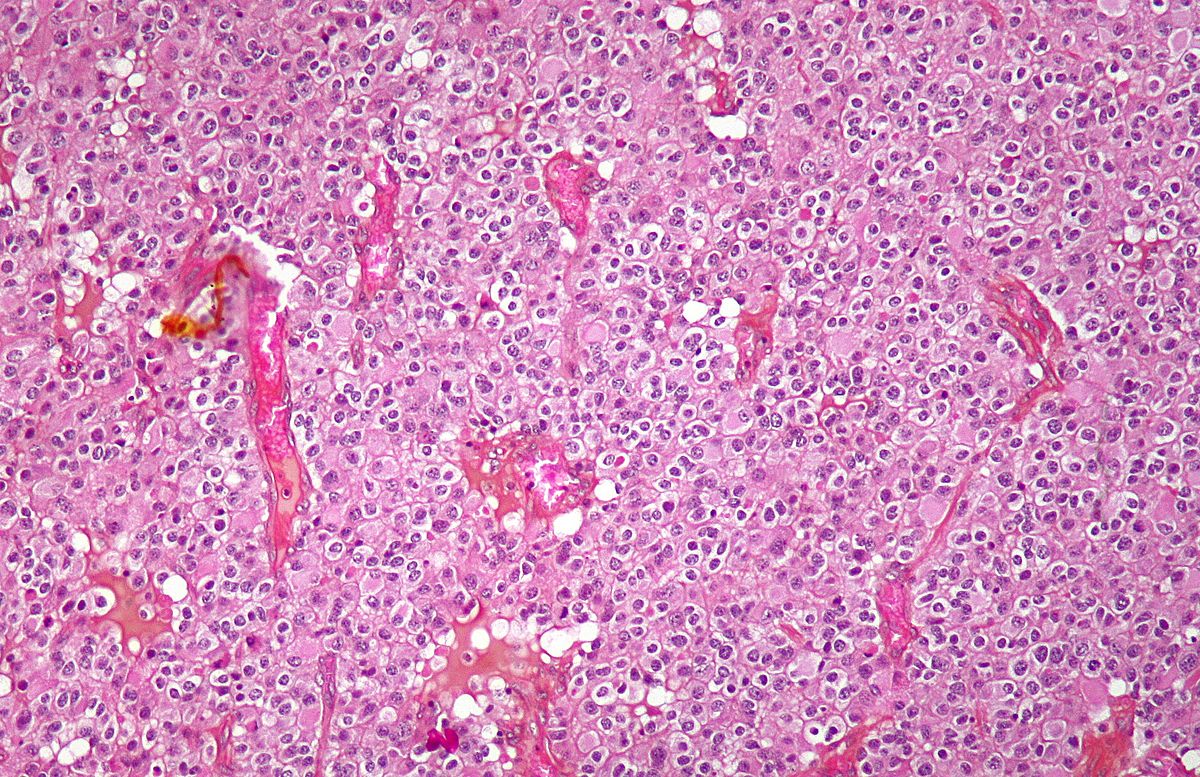
On microscopic histopathological analysis, oligodendroglioma is characterized by:[20][60][61][62][63]
- Diffusely growing, infiltrative tumor
- Moderate cellularity
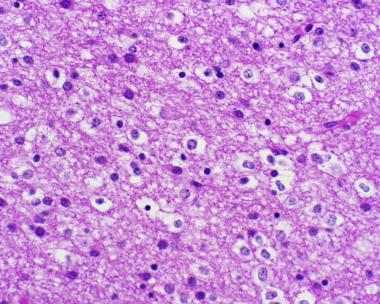
- Highly cellular lesion composed of typically monomorphic cells resembling fried eggs with:
- Round nucleus - key feature
- Distinct cell borders
- Moderate-to-marked nuclear atypia with speckled "salt-and-pepper" chromatin pattern
- Inconspicuous nucleoli
- Clear cytoplasm (artifactual retraction of the cytoplasm on routinely processed formalin fixed, paraffin embedded material, leading to the characteristic "fried egg" appearance)
- Some oligodendrogliomas have eosinophilic cytoplasm with focal perinuclear clearing
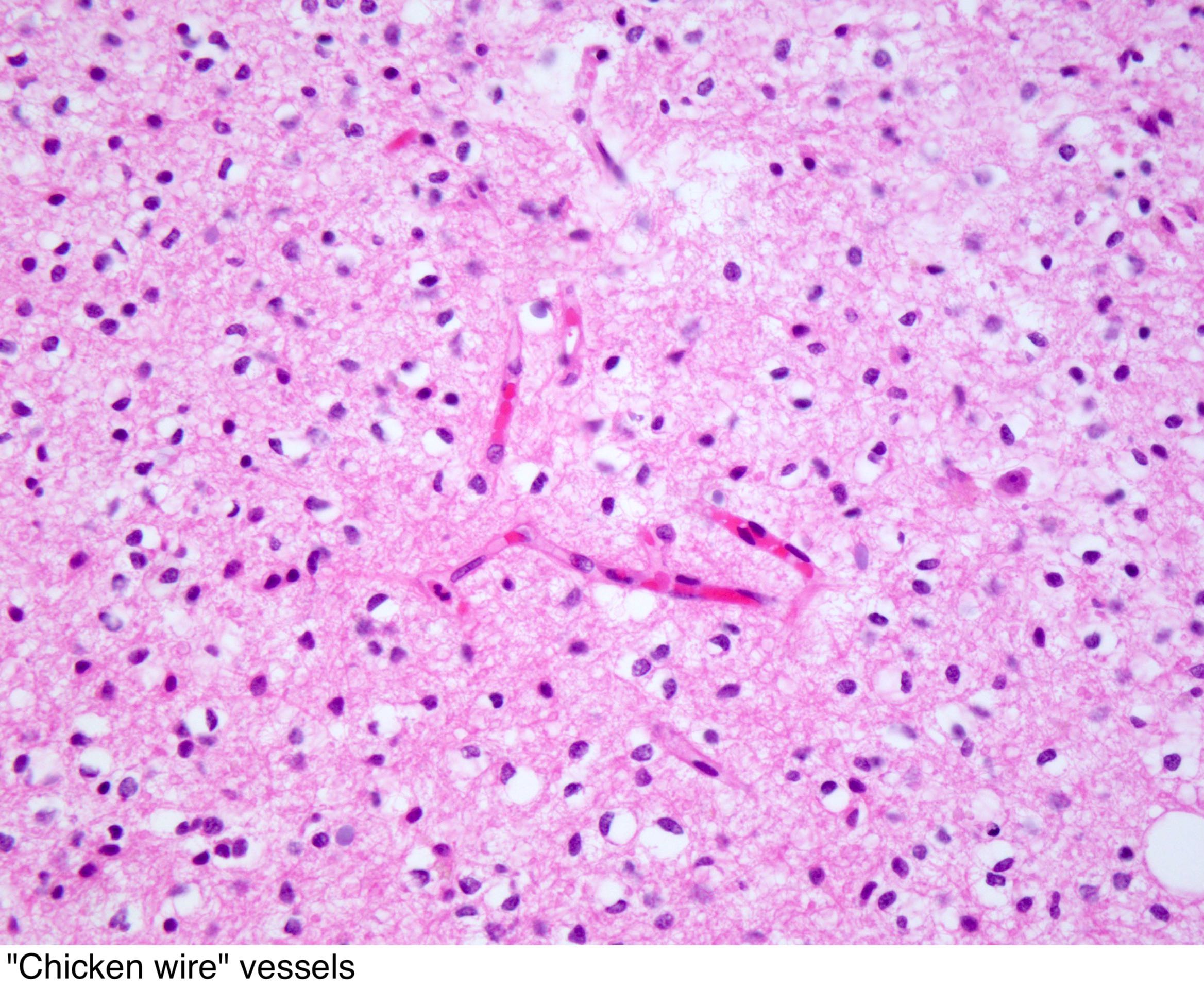
- Dense network of acutely fine branched capillary sized vessels -classically referred to as a "chicken-wire" like appearance/pattern[64]
- Abundant and delicate appearing; may vaguely resemble a paraganglioma at low power
- Small punctate calcifications, particularly along the blood vessels is a striking feature (but not a specific finding)
- Perifocal edema - rare
- Few tumors may exhibit eosinophilic granular bodies
- Some tumors may show a spongioblastoma-like growth pattern
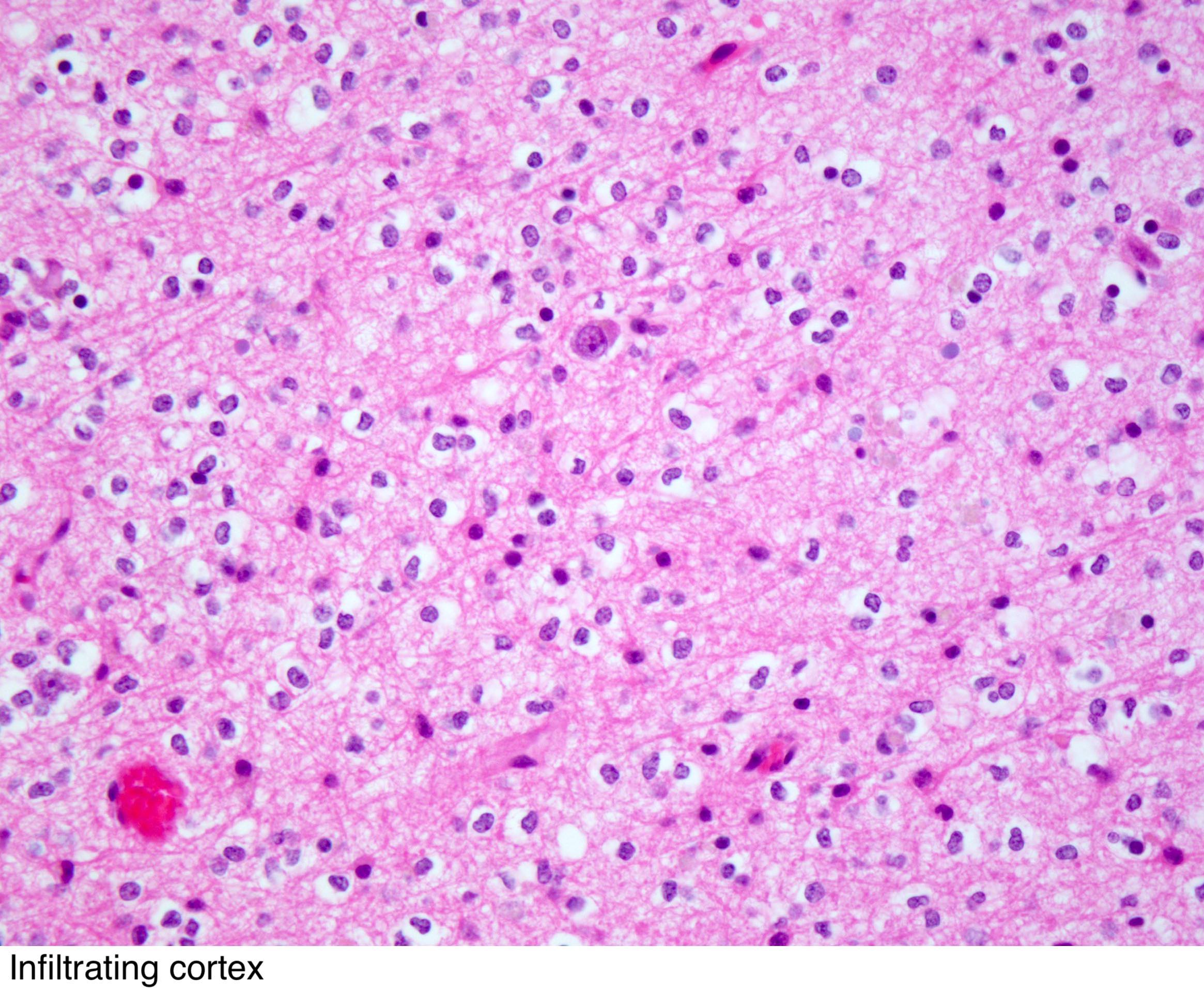
- Tumor cells may form following secondary structures in the surrounding infiltrated brain parenchyma:
- Perineuronal satellitosis
- Subpial accumulation
- Perivascular distribution (less common)
- Microgemistocytic appearance of tumor cells with a rounded belly of eccentric GFAP+ eosinophilic cytoplasm (maybe present)[65]
- A predominant fibrillar astrocytic phenotype is compatible with the diagnosis when following appropriate molecular findings are present:[21]
- IDH mutation
- 1p/19q codeletion
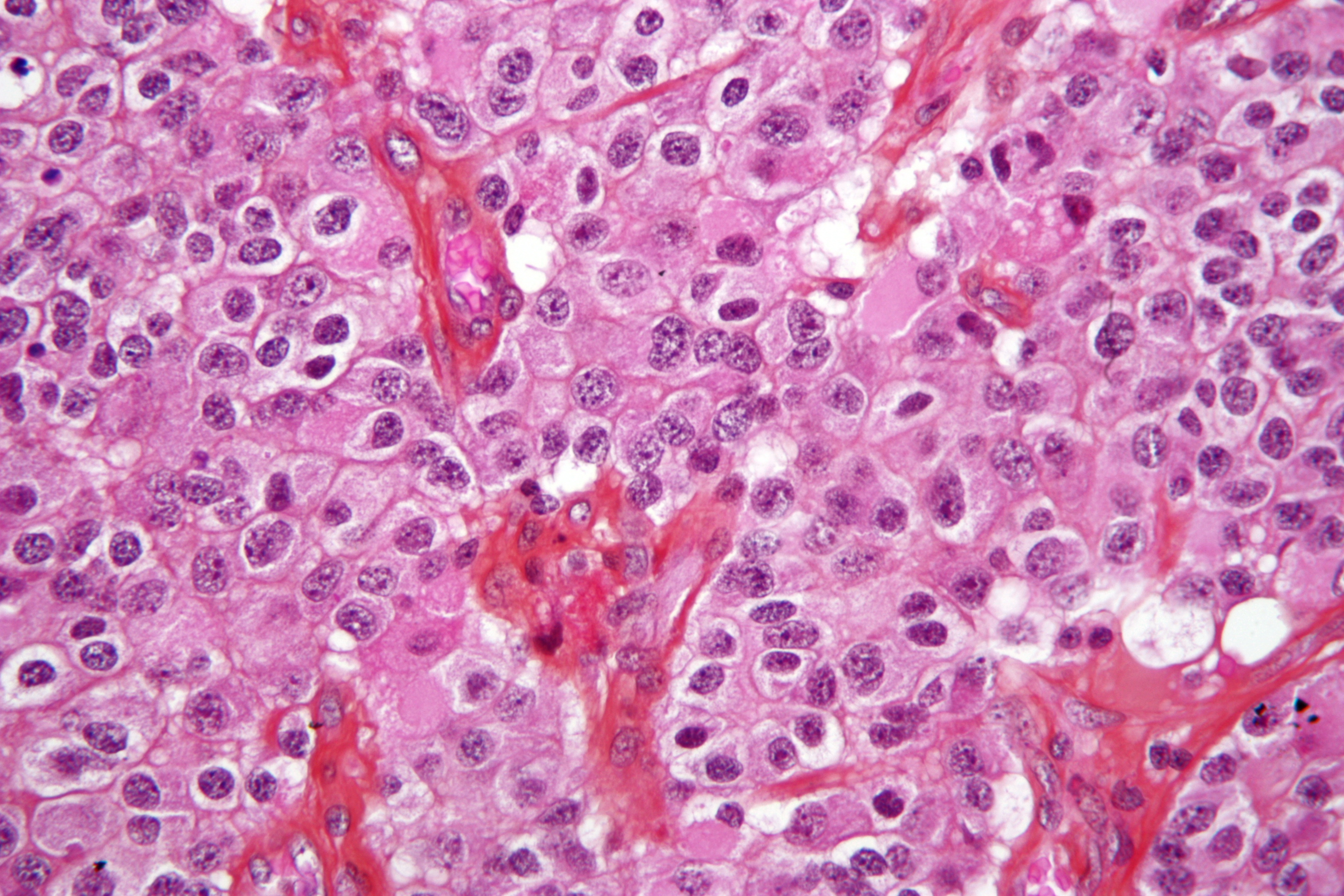
Microscopic histopathological findings in anaplastic oligodendroglioma
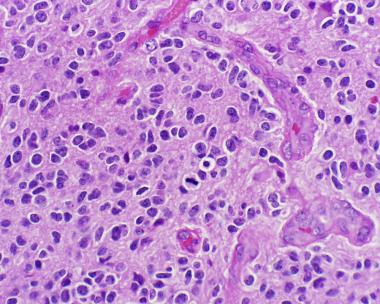
On microscopic histopathological analysis, anaplastic oligodendroglioma, IDH mutant and 1p/19q codeleted, is characterized by:[60]
- Focal or diffusely increased cell density
- Atypical to frankly pleomorphic cells or multinucleated giant cells
- Tumor cells may be plasmacytoid (i.e. have a plasma cell-like appearance)
- Also called as minigemistocytes
- Significant/brisk infrequent mitotic activity (≥ 6 mitoses per 10 HPF)[66][67]
- Rare foci of:
- Necrosis
- Apoptotic cells
- Microvacular proliferation either in the form of:
 |
 |
 |
 |
 |
 |
 |
Immunohistochemistry
Oligodendroglioma is demonstrated by positivity to tumor markers such as:[68][69][20][7]
- IDH1-R132H (majority of cases)[70]
- MAP2
- GFAP (positive in intermingled reactive astrocytes and minigemistocytes)
- SOX10
- S-100
- EMA
- ATRX
- Ki-67
- NSE
- Synaptophysin
- OLIG1
- OLIG2
Oligodendroglioma stains negative for:
- p53 (rare weakly positive cells can be seen)
- Keratins (although cocktails may show cross reactivity)
References
- ↑ General features of oligodendroglioma. Libre Pathology. http://librepathology.org/wiki/index.php/Oligodendroglioma#cite_note-1
- ↑ Suzuki H, Aoki K, Chiba K, Sato Y, Shiozawa Y, Shiraishi Y; et al. (2015). "Mutational landscape and clonal architecture in grade II and III gliomas". Nat Genet. 47 (5): 458–68. doi:10.1038/ng.3273. PMID 25848751.
- ↑ Leeper HE, Caron AA, Decker PA, Jenkins RB, Lachance DH, Giannini C (2015). "IDH mutation, 1p19q codeletion and ATRX loss in WHO grade II gliomas". Oncotarget. 6 (30): 30295–305. doi:10.18632/oncotarget.4497. PMC 4745799. PMID 26210286.
- ↑ Sabha N, Knobbe CB, Maganti M, Al Omar S, Bernstein M, Cairns R; et al. (2014). "Analysis of IDH mutation, 1p/19q deletion, and PTEN loss delineates prognosis in clinical low-grade diffuse gliomas". Neuro Oncol. 16 (7): 914–23. doi:10.1093/neuonc/not299. PMC 4057130. PMID 24470545.
- ↑ Zhang J, Wu G, Miller CP, Tatevossian RG, Dalton JD, Tang B; et al. (2013). "Whole-genome sequencing identifies genetic alterations in pediatric low-grade gliomas". Nat Genet. 45 (6): 602–12. doi:10.1038/ng.2611. PMC 3727232. PMID 23583981.
- ↑ Wu G, Diaz AK, Paugh BS, Rankin SL, Ju B, Li Y; et al. (2014). "The genomic landscape of diffuse intrinsic pontine glioma and pediatric non-brainstem high-grade glioma". Nat Genet. 46 (5): 444–450. doi:10.1038/ng.2938. PMC 4056452. PMID 24705251.
- ↑ 7.0 7.1 Tanboon J, Williams EA, Louis DN (2016). "The Diagnostic Use of Immunohistochemical Surrogates for Signature Molecular Genetic Alterations in Gliomas". J Neuropathol Exp Neurol. 75 (1): 4–18. doi:10.1093/jnen/nlv009. PMID 26671986.
- ↑ Jiao Y, Killela PJ, Reitman ZJ, Rasheed AB, Heaphy CM, de Wilde RF; et al. (2012). "Frequent ATRX, CIC, FUBP1 and IDH1 mutations refine the classification of malignant gliomas". Oncotarget. 3 (7): 709–22. doi:10.18632/oncotarget.588. PMC 3443254. PMID 22869205.
- ↑ Sahm F, Koelsche C, Meyer J, Pusch S, Lindenberg K, Mueller W; et al. (2012). "CIC and FUBP1 mutations in oligodendrogliomas, oligoastrocytomas and astrocytomas". Acta Neuropathol. 123 (6): 853–60. doi:10.1007/s00401-012-0993-5. PMID 22588899.
- ↑ 10.0 10.1 Barbashina V, Salazar P, Holland EC, Rosenblum MK, Ladanyi M (2005). "Allelic losses at 1p36 and 19q13 in gliomas: correlation with histologic classification, definition of a 150-kb minimal deleted region on 1p36, and evaluation of CAMTA1 as a candidate tumor suppressor gene". Clin Cancer Res. 11 (3): 1119–28. PMID 15709179.
- ↑ Frenel JS, Leux C, Loussouarn D, Le Loupp AG, Leclair F, Aumont M; et al. (2013). "Combining two biomarkers, IDH1/2 mutations and 1p/19q codeletion, to stratify anaplastic oligodendroglioma in three groups: a single-center experience". J Neurooncol. 114 (1): 85–91. doi:10.1007/s11060-013-1152-0. PMID 23681562.
- ↑ Hacisalihoglu P, Kucukodaci Z, Gundogdu G, Bilgic B (2017). "The Correlation Between 1p/19q Codeletion, IDH1 Mutation, p53 Overexpression and Their Prognostic Roles in 41 Turkish Anaplastic Oligodendroglioma Patients". Turk Neurosurg. 27 (5): 682–689. doi:10.5137/1019-5149.JTN.16832-15.1. PMID 27651340.
- ↑ Molecular genetics of oligodendroglioma. https://en.wikipedia.org/wiki/Oligodendroglioma
- ↑ Bettegowda C, Agrawal N, Jiao Y, Sausen M, Wood LD, Hruban RH; et al. (2011). "Mutations in CIC and FUBP1 contribute to human oligodendroglioma". Science. 333 (6048): 1453–5. doi:10.1126/science.1210557. PMC 3170506. PMID 21817013.
- ↑ Prognosis and treatment of oligodendroglioma. Wikipedia 2015. https://en.wikipedia.org/wiki/Oligodendroglioma
- ↑ 16.0 16.1 Yip S, Butterfield YS, Morozova O, Chittaranjan S, Blough MD, An J; et al. (2012). "Concurrent CIC mutations, IDH mutations, and 1p/19q loss distinguish oligodendrogliomas from other cancers". J Pathol. 226 (1): 7–16. doi:10.1002/path.2995. PMC 3246739. PMID 22072542.
- ↑ Labreche K, Simeonova I, Kamoun A, Gleize V, Chubb D, Letouzé E; et al. (2015). "TCF12 is mutated in anaplastic oligodendroglioma". Nat Commun. 6: 7207. doi:10.1038/ncomms8207. PMC 4490400. PMID 26068201.
- ↑ Suri V, Jha P, Agarwal S, Pathak P, Sharma MC, Sharma V; et al. (2011). "Molecular profile of oligodendrogliomas in young patients". Neuro Oncol. 13 (10): 1099–106. doi:10.1093/neuonc/nor146. PMC 3177666. PMID 21937591.
- ↑ Hagel C, Laking G, Laas R, Scheil S, Jung R, Milde-Langosch K; et al. (1996). "Demonstration of p53 protein and TP53 gene mutations in oligodendrogliomas". Eur J Cancer. 32A (13): 2242–8. PMID 9038605.
- ↑ 20.0 20.1 20.2 20.3 20.4 von Deimling, A; Hartmann, C (2005). "Oligodendrogliomas: Impact of molecular genetics on treatment". Neurology India. 53 (2): 140. doi:10.4103/0028-3886.16394. ISSN 0028-3886.
- ↑ 21.0 21.1 Cancer Genome Atlas Research Network. Brat DJ, Verhaak RG, Aldape KD, Yung WK, Salama SR; et al. (2015). "Comprehensive, Integrative Genomic Analysis of Diffuse Lower-Grade Gliomas". N Engl J Med. 372 (26): 2481–98. doi:10.1056/NEJMoa1402121. PMC 4530011. PMID 26061751.
- ↑ 22.0 22.1 Eckel-Passow JE, Lachance DH, Molinaro AM, Walsh KM, Decker PA, Sicotte H; et al. (2015). "Glioma Groups Based on 1p/19q, IDH, and TERT Promoter Mutations in Tumors". N Engl J Med. 372 (26): 2499–508. doi:10.1056/NEJMoa1407279. PMC 4489704. PMID 26061753.
- ↑ Ueki K, Nishikawa R, Nakazato Y, Hirose T, Hirato J, Funada N; et al. (2002). "Correlation of histology and molecular genetic analysis of 1p, 19q, 10q, TP53, EGFR, CDK4, and CDKN2A in 91 astrocytic and oligodendroglial tumors". Clin Cancer Res. 8 (1): 196–201. PMID 11801559.
- ↑ Labussière M, Boisselier B, Mokhtari K, Di Stefano AL, Rahimian A, Rossetto M; et al. (2014). "Combined analysis of TERT, EGFR, and IDH status defines distinct prognostic glioblastoma classes". Neurology. 83 (13): 1200–6. doi:10.1212/WNL.0000000000000814. PMID 25150284.
- ↑ Nambirajan A, Suri V, Kedia S, Goyal K, Malgulwar PB, Khanna G; et al. (2018). "Paediatric diffuse leptomeningeal tumor with glial and neuronal differentiation harbouring chromosome 1p/19q co-deletion and H3.3 K27M mutation: unusual molecular profile and its therapeutic implications". Brain Tumor Pathol. 35 (3): 186–191. doi:10.1007/s10014-018-0325-0. PMID 30030640.
- ↑ McDonald JM, See SJ, Tremont IW, Colman H, Gilbert MR, Groves M; et al. (2005). "The prognostic impact of histology and 1p/19q status in anaplastic oligodendroglial tumors". Cancer. 104 (7): 1468–77. doi:10.1002/cncr.21338. PMID 16088966.
- ↑ Reifenberger J, Reifenberger G, Liu L, James CD, Wechsler W, Collins VP (1994). "Molecular genetic analysis of oligodendroglial tumors shows preferential allelic deletions on 19q and 1p". Am J Pathol. 145 (5): 1175–90. PMC 1887413. PMID 7977648.
- ↑ Reuss DE, Sahm F, Schrimpf D, Wiestler B, Capper D, Koelsche C; et al. (2015). "ATRX and IDH1-R132H immunohistochemistry with subsequent copy number analysis and IDH sequencing as a basis for an "integrated" diagnostic approach for adult astrocytoma, oligodendroglioma and glioblastoma". Acta Neuropathol. 129 (1): 133–46. doi:10.1007/s00401-014-1370-3. PMID 25427834.
- ↑ Chen N, Yu T, Gong J, Nie L, Chen X, Zhang M; et al. (2016). "IDH1/2 gene hotspot mutations in central nervous system tumours: analysis of 922 Chinese patients". Pathology. 48 (7): 675–683. doi:10.1016/j.pathol.2016.07.010. PMID 27780605.
- ↑ Zhou YX, Wang JX, Feng M, Sun CM, Sun T, Chen GL; et al. (2012). "Analysis of isocitrate dehydrogenase 1 mutation in 97 patients with glioma". J Mol Neurosci. 47 (3): 442–7. doi:10.1007/s12031-011-9681-5. PMID 22113362.
- ↑ Capper D, Weissert S, Balss J, Habel A, Meyer J, Jäger D; et al. (2010). "Characterization of R132H mutation-specific IDH1 antibody binding in brain tumors". Brain Pathol. 20 (1): 245–54. doi:10.1111/j.1750-3639.2009.00352.x. PMID 19903171.
- ↑ Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W; et al. (2009). "IDH1 and IDH2 mutations in gliomas". N Engl J Med. 360 (8): 765–73. doi:10.1056/NEJMoa0808710. PMC 2820383. PMID 19228619.
- ↑ Balss J, Meyer J, Mueller W, Korshunov A, Hartmann C, von Deimling A (2008). "Analysis of the IDH1 codon 132 mutation in brain tumors". Acta Neuropathol. 116 (6): 597–602. doi:10.1007/s00401-008-0455-2. PMID 18985363.
- ↑ Arita H, Narita Y, Matsushita Y, Fukushima S, Yoshida A, Takami H; et al. (2015). "Development of a robust and sensitive pyrosequencing assay for the detection of IDH1/2 mutations in gliomas". Brain Tumor Pathol. 32 (1): 22–30. doi:10.1007/s10014-014-0186-0. PMID 24748374.
- ↑ Setty P, Hammes J, Rothämel T, Vladimirova V, Kramm CM, Pietsch T; et al. (2010). "A pyrosequencing-based assay for the rapid detection of IDH1 mutations in clinical samples". J Mol Diagn. 12 (6): 750–6. doi:10.2353/jmoldx.2010.090237. PMC 2963913. PMID 20847279.
- ↑ Pang B, Durso MB, Hamilton RL, Nikiforova MN (2013). "A novel COLD-PCR/FMCA assay enhances the detection of low-abundance IDH1 mutations in gliomas". Diagn Mol Pathol. 22 (1): 28–34. doi:10.1097/PDM.0b013e31826c7ff8. PMID 23370430.
- ↑ Boisselier B, Marie Y, Labussière M, Ciccarino P, Desestret V, Wang X; et al. (2010). "COLD PCR HRM: a highly sensitive detection method for IDH1 mutations". Hum Mutat. 31 (12): 1360–5. doi:10.1002/humu.21365. PMID 20886613.
- ↑ Pan Y, Qi XL, Wang LM, Dong RF, Zhang M, Zheng DF; et al. (2013). "[Mutation of isocitrate dehydrogenase gene in Chinese patients with glioma]". Zhonghua Bing Li Xue Za Zhi. 42 (5): 292–8. doi:10.3760/cma.j.issn.0529-5807.2013.05.002. PMID 24004584.
- ↑ Hartmann C, Meyer J, Balss J, Capper D, Mueller W, Christians A; et al. (2009). "Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas". Acta Neuropathol. 118 (4): 469–74. doi:10.1007/s00401-009-0561-9. PMID 19554337.
- ↑ Sonoda Y, Kumabe T, Nakamura T, Saito R, Kanamori M, Yamashita Y; et al. (2009). "Analysis of IDH1 and IDH2 mutations in Japanese glioma patients". Cancer Sci. 100 (10): 1996–8. doi:10.1111/j.1349-7006.2009.01270.x. PMID 19765000.
- ↑ Arita H, Narita Y, Yoshida A, Hashimoto N, Yoshimine T, Ichimura K (2015). "IDH1/2 mutation detection in gliomas". Brain Tumor Pathol. 32 (2): 79–89. doi:10.1007/s10014-014-0197-x. PMID 25008158.
- ↑ van den Bent MJ, Dubbink HJ, Marie Y, Brandes AA, Taphoorn MJ, Wesseling P; et al. (2010). "IDH1 and IDH2 mutations are prognostic but not predictive for outcome in anaplastic oligodendroglial tumors: a report of the European Organization for Research and Treatment of Cancer Brain Tumor Group". Clin Cancer Res. 16 (5): 1597–604. doi:10.1158/1078-0432.CCR-09-2902. PMID 20160062.
- ↑ Catteau A, Girardi H, Monville F, Poggionovo C, Carpentier S, Frayssinet V; et al. (2014). "A new sensitive PCR assay for one-step detection of 12 IDH1/2 mutations in glioma". Acta Neuropathol Commun. 2: 58. doi:10.1186/2051-5960-2-58. PMC 4229941. PMID 24889502.
- ↑ Diplas BH, Liu H, Yang R, Hansen LJ, Zachem AL, Zhao F; et al. (2019). "Sensitive and rapid detection of TERT promoter and IDH mutations in diffuse gliomas". Neuro Oncol. 21 (4): 440–450. doi:10.1093/neuonc/noy167. PMC 6422442. PMID 30346624.
- ↑ Sun ZL, Chan AK, Chen LC, Tang C, Zhang ZY, Ding XJ; et al. (2015). "TERT promoter mutated WHO grades II and III gliomas are located preferentially in the frontal lobe and avoid the midline". Int J Clin Exp Pathol. 8 (9): 11485–94. PMC 4637696. PMID 26617880.
- ↑ Yang P, Cai J, Yan W, Zhang W, Wang Y, Chen B; et al. (2016). "Classification based on mutations of TERT promoter and IDH characterizes subtypes in grade II/III gliomas". Neuro Oncol. 18 (8): 1099–108. doi:10.1093/neuonc/now021. PMC 4933482. PMID 26957363.
- ↑ Labussière M, Di Stefano AL, Gleize V, Boisselier B, Giry M, Mangesius S; et al. (2014). "TERT promoter mutations in gliomas, genetic associations and clinico-pathological correlations". Br J Cancer. 111 (10): 2024–32. doi:10.1038/bjc.2014.538. PMC 4229642. PMID 25314060.
- ↑ Nencha U, Rahimian A, Giry M, Sechi A, Mokhtari K, Polivka M; et al. (2016). "TERT promoter mutations and rs2853669 polymorphism: prognostic impact and interactions with common alterations in glioblastomas". J Neurooncol. 126 (3): 441–6. doi:10.1007/s11060-015-1999-3. PMID 26608520.
- ↑ Khuong-Quang DA, Buczkowicz P, Rakopoulos P, Liu XY, Fontebasso AM, Bouffet E; et al. (2012). "K27M mutation in histone H3.3 defines clinically and biologically distinct subgroups of pediatric diffuse intrinsic pontine gliomas". Acta Neuropathol. 124 (3): 439–47. doi:10.1007/s00401-012-0998-0. PMC 3422615. PMID 22661320.
- ↑ Harutyunyan AS, Krug B, Chen H, Papillon-Cavanagh S, Zeinieh M, De Jay N; et al. (2019). "H3K27M induces defective chromatin spread of PRC2-mediated repressive H3K27me2/me3 and is essential for glioma tumorigenesis". Nat Commun. 10 (1): 1262. doi:10.1038/s41467-019-09140-x. PMC 6425035. PMID 30890717.
- ↑ Wu G, Broniscer A, McEachron TA, Lu C, Paugh BS, Becksfort J; et al. (2012). "Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas". Nat Genet. 44 (3): 251–3. doi:10.1038/ng.1102. PMC 3288377. PMID 22286216.
- ↑ Castel D, Philippe C, Calmon R, Le Dret L, Truffaux N, Boddaert N; et al. (2015). "Histone H3F3A and HIST1H3B K27M mutations define two subgroups of diffuse intrinsic pontine gliomas with different prognosis and phenotypes". Acta Neuropathol. 130 (6): 815–27. doi:10.1007/s00401-015-1478-0. PMC 4654747. PMID 26399631.
- ↑ Castel D, Grill J, Debily MA (2016). "Histone H3 genotyping refines clinico-radiological diagnostic and prognostic criteria in DIPG". Acta Neuropathol. 131 (5): 795–6. doi:10.1007/s00401-016-1568-7. PMC 4835508. PMID 27038188.
- ↑ Cordero FJ, Huang Z, Grenier C, He X, Hu G, McLendon RE; et al. (2017). "Histone H3.3K27M Represses p16 to Accelerate Gliomagenesis in a Murine Model of DIPG". Mol Cancer Res. 15 (9): 1243–1254. doi:10.1158/1541-7786.MCR-16-0389. PMC 5581686. PMID 28522693.
- ↑ Eisenreich S, Abou-El-Ardat K, Szafranski K, Campos Valenzuela JA, Rump A, Nigro JM; et al. (2013). "Novel CIC point mutations and an exon-spanning, homozygous deletion identified in oligodendroglial tumors by a comprehensive genomic approach including transcriptome sequencing". PLoS One. 8 (9): e76623. doi:10.1371/journal.pone.0076623. PMC 3785522. PMID 24086756.
- ↑ Gillet E, Alentorn A, Doukouré B, Mundwiller E, van Thuijl HF, van Thuij H; et al. (2014). "TP53 and p53 statuses and their clinical impact in diffuse low grade gliomas". J Neurooncol. 118 (1): 131–9. doi:10.1007/s11060-014-1407-4. PMID 24590827.
- ↑ Rodriguez FJ, Schniederjan MJ, Nicolaides T, Tihan T, Burger PC, Perry A (2015). "High rate of concurrent BRAF-KIAA1549 gene fusion and 1p deletion in disseminated oligodendroglioma-like leptomeningeal neoplasms (DOLN)". Acta Neuropathol. 129 (4): 609–610. doi:10.1007/s00401-015-1400-9. PMC 4696044. PMID 25720745.
- ↑ 58.0 58.1 Gross appearance of oligodendroglioma. Dr Henry Knipe and Dr Frank Gaillard et al. http://radiopaedia.org/articles/oligodendroglioma
- ↑ Gross/radiologic findings of oligodendroglioma. Libre Pathology. http://librepathology.org/wiki/index.php/Oligodendroglioma
- ↑ 60.0 60.1 Microscopic features of oligodendroglioma. Libre Pathology. http://librepathology.org/wiki/index.php/Oligodendroglioma
- ↑ Ersen, Ayca (2008), Pathology of malignant gliomas: Challenges of everyday practice and the WHO 2007, Turkish Journal of Pathology, retrieved 9 October, 2015 Check date values in:
|accessdate=(help) - ↑ Eskandar EN, Loeffler JS, O'Neill AM, Hunter GJ, Louis DN (2004). "Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 33-2004. A 34-year-old man with a seizure and a frontal-lobe brain lesion". N Engl J Med. 351 (18): 1875–82. doi:10.1056/NEJMcpc049025. PMID 15509821.
- ↑ Rodriguez FJ, Perry A, Rosenblum MK, Krawitz S, Cohen KJ, Lin D; et al. (2012). "Disseminated oligodendroglial-like leptomeningeal tumor of childhood: a distinctive clinicopathologic entity". Acta Neuropathol. 124 (5): 627–41. doi:10.1007/s00401-012-1037-x. PMID 22941225.
- ↑ Images of microscopic appearance of oligodendroglioma. Wikipedia 2015. https://en.wikipedia.org/wiki/Oligodendroglioma
- ↑ Kros JM, Van Eden CG, Stefanko SZ, Waayer-Van Batenburg M, van der Kwast TH (1990). "Prognostic implications of glial fibrillary acidic protein containing cell types in oligodendrogliomas". Cancer. 66 (6): 1204–12. PMID 2205356.
- ↑ Images of oligodendroglioma. Libre Pathology 2015. http://librepathology.org/wiki/index.php/Oligodendroglioma
- ↑ Smith SF, Simpson JM, Brewer JA, Sekhon LH, Biggs MT, Cook RJ; et al. (2006). "The presence of necrosis and/or microvascular proliferation does not influence survival of patients with anaplastic oligodendroglial tumours: review of 98 patients". J Neurooncol. 80 (1): 75–82. doi:10.1007/s11060-006-9158-5. PMID 16794749.
- ↑ IHC of oligodendroglioma. Libre Pathology. http://librepathology.org/wiki/index.php/Oligodendroglioma
- ↑ Hilbig A, Barbosa-Coutinho LM, Netto GC, Bleil CB, Toscani NV (2006). "[Immunohistochemistry in oligodendrogliomas]". Arq Neuropsiquiatr. 64 (1): 67–71. doi:/S0004-282X2006000100014 Check
|doi=value (help). PMID 16622556. - ↑ Kato Y (2015). "Specific monoclonal antibodies against IDH1/2 mutations as diagnostic tools for gliomas". Brain Tumor Pathol. 32 (1): 3–11. doi:10.1007/s10014-014-0202-4. PMID 25324168.