SandboxAlonso: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
{{Timolol}} | |||
{{Drugbox | |||
| verifiedrevid = 457286138 | |||
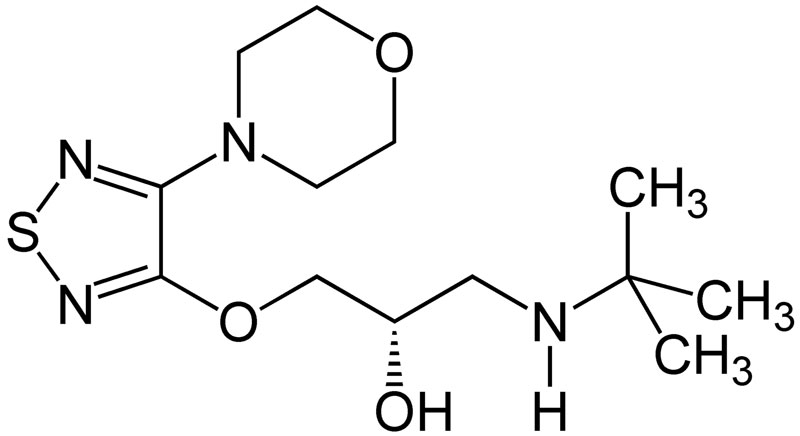
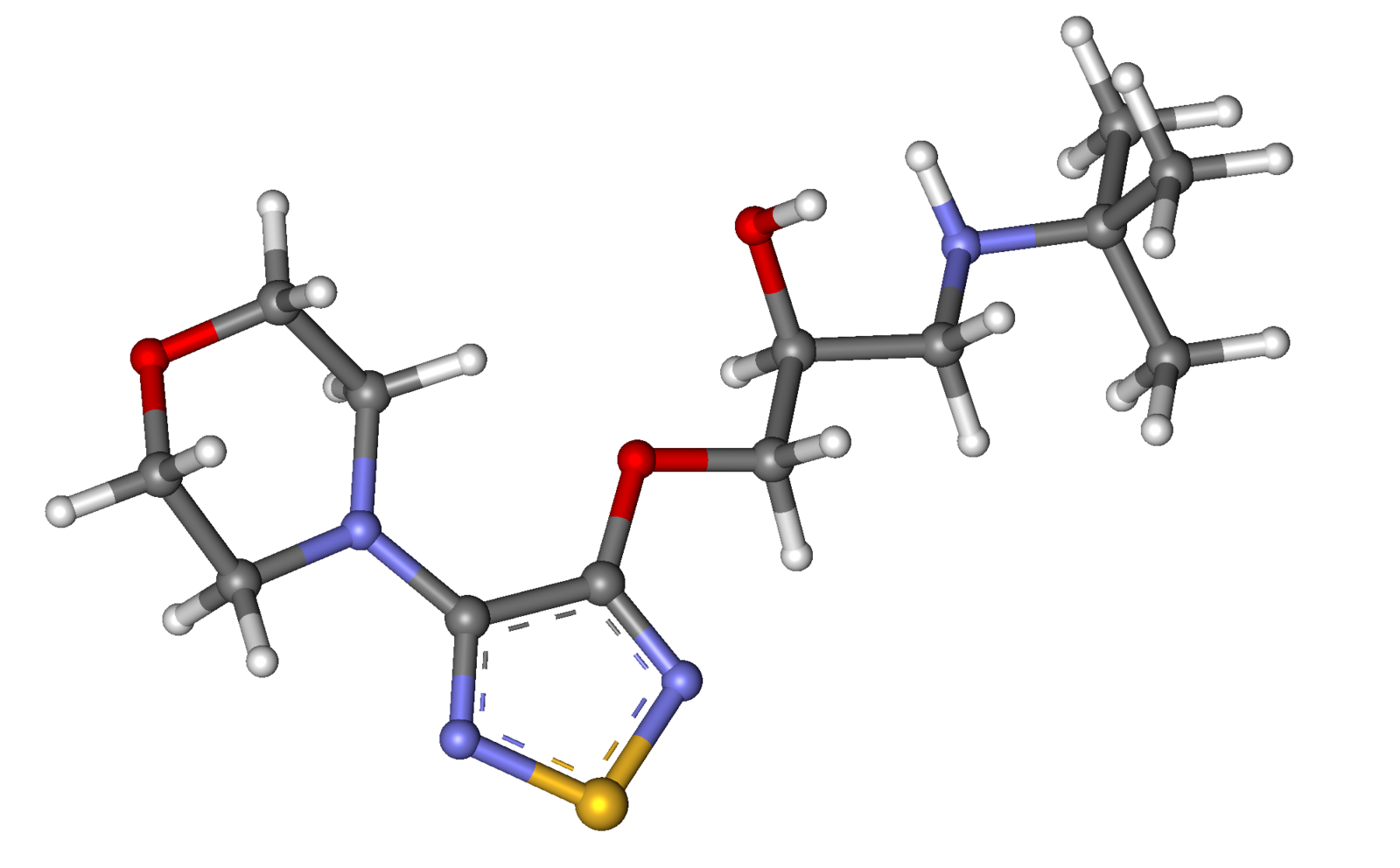
| IUPAC_name = (''S'')-1-(''tert''-butylamino)-3-[(4-morpholin-4-yl-1,2,5-thiadiazol-3-yl)oxy]propan-2-ol | |||
| image = Timol01.jpg | |||
| image2 =Timolol02.png | |||
<!--Clinical data--> | |||
| tradename = Timoptic | |||
| Drugs.com = {{drugs.com|monograph|timolol-eent}} | |||
| MedlinePlus = a602022 | |||
| pregnancy_AU = C | |||
| pregnancy_US = C | |||
| legal_status = Rx-only | |||
| routes_of_administration = oral, [[human eye|Ophthalmic]] | |||
<!--Pharmacokinetic data--> | |||
| bioavailability = 60% | |||
| metabolism = [[Liver|Hepatic]]: 80% | |||
| elimination_half-life = 2.5-5 hours | |||
| excretion = [[Kidney|Renal]] | |||
<!--Identifiers--> | |||
| CASNo_Ref = {{cascite|correct|CAS}} | |||
| CAS_number_Ref = {{cascite|correct|??}} | |||
| CAS_number = 26839-75-8 | |||
| ATC_prefix = C07 | |||
| ATC_suffix = AA06 | |||
| ATC_supplemental = {{ATC|S01|ED01}} | |||
| PubChem = 33624 | |||
| IUPHAR_ligand = 565 | |||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| DrugBank = DB00373 | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 31013 | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = 5JKY92S7BR | |||
| KEGG_Ref = {{keggcite|correct|kegg}} | |||
| KEGG = D08600 | |||
| ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| ChEBI = 9599 | |||
| ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| ChEMBL = 499 | |||
<!--Chemical data--> | |||
| C=13 | H=24 | N=4 | O=3 | S=1 | |||
| molecular_weight = 316.421 g/mol | |||
| smiles = O[C@H](COc1nsnc1N2CCOCC2)CNC(C)(C)C | |||
| InChI = 1/C13H24N4O3S/c1-13(2,3)14-8-10(18)9-20-12-11(15-21-16-12)17-4-6-19-7-5-17/h10,14,18H,4-9H2,1-3H3/t10-/m0/s1 | |||
| InChIKey = BLJRIMJGRPQVNF-JTQLQIEIBV | |||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChI = 1S/C13H24N4O3S/c1-13(2,3)14-8-10(18)9-20-12-11(15-21-16-12)17-4-6-19-7-5-17/h10,14,18H,4-9H2,1-3H3/t10-/m0/s1 | |||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChIKey = BLJRIMJGRPQVNF-JTQLQIEISA-N | |||
}} | |||
__NOTOC__ | __NOTOC__ | ||
{{CMG}} | |||
{{CMG | |||
'''''For patient information about | '''''For patient information about Timolol Tablet, click [[Timolol Oral (patient information)|here]].''''' | ||
{{SB}} | '''''For patient information about Timolol Drop/Solution, click [[Timolol Ophthalmic (patient information)|here]].''''' | ||
{{SB}} Betimol, Blocadren, Istalol, Timoptic, Timoptic-XE, Timoptic OcuDose | |||
==Overview== | ==Overview== | ||
''' | '''Timolol maleate''' is a non-selective [[beta blocker|beta-adrenergic receptor antagonist]] indicated for treating [[glaucoma]], [[myocardial infarction|heart attacks]] and [[hypertension]]. | ||
==Category== | ==Category== | ||
Beta-blockers | |||
==FDA Package Insert== | ==FDA Package Insert== | ||
''' | ====TIMOLOL MALEATE tablet==== | ||
'''| [[ | |||
'''| [[ | ''' [[Timolol tablet indications and usage|Indications and Usage]]''' | ||
'''| [[ | '''| [[Timolol tablet dosage and administration|Dosage and Administration]]''' | ||
'''| [[ | '''| [[Timolol tablet contraindications|Contraindications]]''' | ||
'''| [[ | '''| [[Timolol tablet warnings and precautions|Warnings and Precautions]]''' | ||
'''| [[ | '''| [[Timolol tablet adverse reactions|Adverse Reactions]]''' | ||
'''| [[ | '''| [[Timolol tablet drug interactions|Drug Interactions]]''' | ||
'''| [[ | '''| [[Timolol tablet use in specific populations|Use in Specific Populations]]''' | ||
'''| [[ | '''| [[Timolol tablet overdosage|Overdosage]]''' | ||
'''| [[ | '''| [[Timolol tablet description|Description]]''' | ||
'''| [[ | '''| [[Timolol tablet clinical pharmacology|Clinical Pharmacology]]''' | ||
'''| [[ | '''| [[Timolol tablet how supplied storage and handling|How Supplied/Storage and Handling]]''' | ||
'''| [[ | '''| [[Timolol tablet labels and packages|Labels and Packages]]''' | ||
{| | |||
| [[File:Ti01.png|800px|thumb]] | |||
|} | |||
==Uses== | |||
In its oral form ('''Blocadren'''), it is used: | |||
* to treat [[hypertension|high blood pressure]] | |||
* to prevent [[myocardial infarction|heart attack]]s | |||
* to prevent [[migraine]] headaches<ref name="MarcusBain2009">{{cite book|author1=Dawn A. Marcus|author2=Philip A. Bain|title=Effective Migraine Treatment in Pregnant and Lactating Women: A Practical Guide|url=http://books.google.com/books?id=Z5YWpjj89NEC&pg=PA141|accessdate=14 November 2010|date=27 February 2009|publisher=シュプリンガー・ジャパン株式会社|isbn=978-1-60327-438-8|pages=141–}}</ref> | |||
In its [[human eye|ophthalmic]] form (brand names '''Timoptol''' in Italy; '''Timoptic'''), it is used to treat open-angle and occasionally secondary [[glaucoma]] by reducing aqueous humour production through blockage of the beta receptors on the ciliary epithelium. The pharmacological mechanism by which it actually does this is still unknown. First beta-blocker approved for topical use in treatment of glaucoma in the USA (1978). With monotherapy, depresses [[Intraocular pressure|IOP]] 18-34% below baseline within first few treatments. However, there are short-term escape and long-term drift effects in some patients. That is, tolerance develops. May reduce extent of diurnal IOP curve up to 50%. IOP higher during sleep. 5-10x more potent beta-blocker than propranolol. Light sensitive; preserved with 0.01% benzalkonium Cl (and also comes BAC free). Can also be used in adjunctive therapy with pilocarpine or CAIs. | |||
==Side effects== | |||
The most serious possible side effects include cardiac [[arrhythmia]]s and severe [[bronchospasm]]s. Timolol can also lead to [[Syncope (medicine)|fainting]], [[congestive heart failure]], [[clinical depression|depression]], [[confusion]], worsening of [[Raynaud's syndrome]] and [[impotence]]. | |||
==Usual dosage== | |||
*Children and Adults: Ophthalmic: Initial: 0.25% solution, instill 1 drop twice daily; increase to 0.5% solution if response not adequate; decrease to 1 drop/day if *controlled; do not exceed 2 drops twice daily of 0.5% solution Adults: Oral: | |||
*Hypertension: Initial: 10 mg twice daily, increase gradually every 14 days, usual dosage: 20–40 mg/day in 2 divided doses; maximum: 60 mg/day | |||
*Prevention of myocardial infarction: 10 mg twice daily initiated within 1–6 weeks after infarction | |||
*Migraine headache: Initial: 10 mg twice daily, increase to maximum of 30 mg/day | |||
==Formulations== | |||
*Gel-forming solution, ophthalmic, as maleate (Timoptic-XE): 0.25% (2.5 mL, 5 mL); 0.5% (2.5 mL, 5 mL) | |||
*Solution, ophthalmic, as hemihydrate (Betimol): 0.25% (5 mL, 10 mL, 15 mL); 0.5% (5 mL, 10 mL, 15 mL) [contains [[benzalkonium chloride]]] | |||
*Solution, ophthalmic, as maleate: 0.25% (5 mL, 10 mL, 15 mL); 0.5% (5 mL, 10 mL, 15 mL) [contains benzalkonium chloride] | |||
*Timoptic: 0.25% (5 mL, 10 mL); 0.5% (5 mL, 10 mL) [contains benzalkonium chloride] | |||
*Solution, ophthalmic, as maleate [preservative free] (Timoptic OcuDose): 0.25% (0.2 mL);0.5% (0.2 mL) [single use] | |||
*Tablet, as maleate (Blocadren): 5 mg, 10 mg, 20 mg | |||
For ophthalmic use, timolol is also available combined with other medications: | |||
*[[Combigan]] - timolol and [[brimonidine]] | |||
*IOTIM-B - timolol and brimonidine | |||
*[[Cosopt]] - timolol maleate and [[Dorzolamide|dorzolamide hydrochloride]] | |||
*[[Alcon#Glaucoma|DuoTrav]] - timolol and [[travoprost]] | |||
*XOalacom (Pfizer) - timolol and latanoprost | |||
==Brand names== | |||
* In Canada: Apo-Timol, Apo-Timop, Gen-Timolol, Nu-Timolol, Phoxal-timolol, PMS-Timolol, Tim-AK, Timoptic, Timoptic-XE. | |||
* In the United States: Betimol, Blocadren, Istalol, Timoptic, Timoptic-XE, Timoptic OcuDose. | |||
* In Jordan: Apimol. | |||
* IOTIM is No. 1 prescribed brand in INDIA from FDC LIMITED. | |||
==Chemical synthesis== | |||
{| | |||
| [[File:Timolol03.png|800px|thumb]] | |||
|} | |||
==Mechanism of Action== | ==Mechanism of Action== | ||
Timolol maleate is a beta1 and beta2 (nonselective) adrenergic receptor blocking agent that does not have significant intrinsic sympathomimetic, direct myocardial depressant, or local anesthetic activity. | |||
==References== | ==References== | ||
{{Reflist|2}} | |||
{{ | {{Beta blockers}} | ||
{{Antiglaucoma preparations and miotics}} | |||
[[Category:Beta blockers]] | |||
[[Category:Morpholines]] | |||
[[Category:Cardiovascular Drugs]] | [[Category:Cardiovascular Drugs]] | ||
[[Category:Antimigraine drugs]] | |||
[[Category:Drugs]] | [[Category:Drugs]] | ||
Revision as of 13:03, 14 July 2014
| Timolol |
|---|
| TIMOLOL MALEATE® FDA Package Insert |
| Indications and Usage |
| Dosage and Administration |
| Contraindications |
| Warnings and Precautions |
| Adverse Reactions |
| Drug Interactions |
| Use in Specific Populations |
| Overdosage |
| Description |
| Clinical Pharmacology |
| How Supplied/Storage and Handling |
| Labels and Packages |
| Clinical Trials on Timolol |
| ClinicalTrials.gov |
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Timoptic |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a602022 |
| Pregnancy category | |
| Routes of administration | oral, Ophthalmic |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 60% |
| Metabolism | Hepatic: 80% |
| Elimination half-life | 2.5-5 hours |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C13H24N4O3S |
| Molar mass | 316.421 g/mol |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
For patient information about Timolol Tablet, click here.
For patient information about Timolol Drop/Solution, click here.
Synonyms / Brand Names: Betimol, Blocadren, Istalol, Timoptic, Timoptic-XE, Timoptic OcuDose
Overview
Timolol maleate is a non-selective beta-adrenergic receptor antagonist indicated for treating glaucoma, heart attacks and hypertension.
Category
Beta-blockers
FDA Package Insert
TIMOLOL MALEATE tablet
Indications and Usage | Dosage and Administration | Contraindications | Warnings and Precautions | Adverse Reactions | Drug Interactions | Use in Specific Populations | Overdosage | Description | Clinical Pharmacology | How Supplied/Storage and Handling | Labels and Packages
 |
Uses
In its oral form (Blocadren), it is used:
- to treat high blood pressure
- to prevent heart attacks
- to prevent migraine headaches[1]
In its ophthalmic form (brand names Timoptol in Italy; Timoptic), it is used to treat open-angle and occasionally secondary glaucoma by reducing aqueous humour production through blockage of the beta receptors on the ciliary epithelium. The pharmacological mechanism by which it actually does this is still unknown. First beta-blocker approved for topical use in treatment of glaucoma in the USA (1978). With monotherapy, depresses IOP 18-34% below baseline within first few treatments. However, there are short-term escape and long-term drift effects in some patients. That is, tolerance develops. May reduce extent of diurnal IOP curve up to 50%. IOP higher during sleep. 5-10x more potent beta-blocker than propranolol. Light sensitive; preserved with 0.01% benzalkonium Cl (and also comes BAC free). Can also be used in adjunctive therapy with pilocarpine or CAIs.
Side effects
The most serious possible side effects include cardiac arrhythmias and severe bronchospasms. Timolol can also lead to fainting, congestive heart failure, depression, confusion, worsening of Raynaud's syndrome and impotence.
Usual dosage
- Children and Adults: Ophthalmic: Initial: 0.25% solution, instill 1 drop twice daily; increase to 0.5% solution if response not adequate; decrease to 1 drop/day if *controlled; do not exceed 2 drops twice daily of 0.5% solution Adults: Oral:
- Hypertension: Initial: 10 mg twice daily, increase gradually every 14 days, usual dosage: 20–40 mg/day in 2 divided doses; maximum: 60 mg/day
- Prevention of myocardial infarction: 10 mg twice daily initiated within 1–6 weeks after infarction
- Migraine headache: Initial: 10 mg twice daily, increase to maximum of 30 mg/day
Formulations
- Gel-forming solution, ophthalmic, as maleate (Timoptic-XE): 0.25% (2.5 mL, 5 mL); 0.5% (2.5 mL, 5 mL)
- Solution, ophthalmic, as hemihydrate (Betimol): 0.25% (5 mL, 10 mL, 15 mL); 0.5% (5 mL, 10 mL, 15 mL) [contains benzalkonium chloride]
- Solution, ophthalmic, as maleate: 0.25% (5 mL, 10 mL, 15 mL); 0.5% (5 mL, 10 mL, 15 mL) [contains benzalkonium chloride]
- Timoptic: 0.25% (5 mL, 10 mL); 0.5% (5 mL, 10 mL) [contains benzalkonium chloride]
- Solution, ophthalmic, as maleate [preservative free] (Timoptic OcuDose): 0.25% (0.2 mL);0.5% (0.2 mL) [single use]
- Tablet, as maleate (Blocadren): 5 mg, 10 mg, 20 mg
For ophthalmic use, timolol is also available combined with other medications:
- Combigan - timolol and brimonidine
- IOTIM-B - timolol and brimonidine
- Cosopt - timolol maleate and dorzolamide hydrochloride
- DuoTrav - timolol and travoprost
- XOalacom (Pfizer) - timolol and latanoprost
Brand names
- In Canada: Apo-Timol, Apo-Timop, Gen-Timolol, Nu-Timolol, Phoxal-timolol, PMS-Timolol, Tim-AK, Timoptic, Timoptic-XE.
- In the United States: Betimol, Blocadren, Istalol, Timoptic, Timoptic-XE, Timoptic OcuDose.
- In Jordan: Apimol.
- IOTIM is No. 1 prescribed brand in INDIA from FDC LIMITED.
Chemical synthesis
 |
Mechanism of Action
Timolol maleate is a beta1 and beta2 (nonselective) adrenergic receptor blocking agent that does not have significant intrinsic sympathomimetic, direct myocardial depressant, or local anesthetic activity.
References
- ↑ Dawn A. Marcus; Philip A. Bain (27 February 2009). Effective Migraine Treatment in Pregnant and Lactating Women: A Practical Guide. シュプリンガー・ジャパン株式会社. pp. 141–. ISBN 978-1-60327-438-8. Retrieved 14 November 2010.