Sandbox:Cherry: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
{{#ev:youtube|https://youtu.be/7XLKn5G_GeA}} | |||
https://www.youtube.com/watch?v=7XLKn5G_GeA | https://www.youtube.com/watch?v=7XLKn5G_GeA | ||
===5-Year Survival=== | ===5-Year Survival=== | ||
Revision as of 18:50, 15 November 2017
{{#ev:youtube|https://youtu.be/7XLKn5G_GeA}}
https://www.youtube.com/watch?v=7XLKn5G_GeA
5-Year Survival
- For patients with localized disease and small cancers (<2 cm) with no lymph node metastases and no extension beyond the capsule of the pancreas, complete surgical resection is associated with a 5-year survival rate of 18% to 24%.
- Between 2007 and 2010, the 5-year relative survival of patients with pancreatic cancer was 7.2%.[1]
- When stratified by age, the 5-year relative survival of patients with pancreatic cancer was 10% and 4.6% for patients <65 and ≥ 65 years of age respectively.[1]
- The survival of patients with pancreatic cancer varies with the stage of the disease. Shown below is a table depicting the 5-year relative survival by the stage of pancreatic cancer:[1][2]
| Stage | 5-year relative survival (%), (2004-2010) |
| All stages | 6.7% |
| Localized | 25.8% |
| Regional | 9.9% |
| Distant | 2.3% |
| Unstaged | 4.4% |
Shown below is an image depicting the 5-year conditional relative survival (probability of surviving in the next 5-years given the cohort has already survived 0, 1, 3 years) between 1988 and 2010 of pancreatic cancer by stage at diagnosis according to SEER. These graphs are adapted from SEER: The Surveillance, Epidemiology, and End Results Program of the National Cancer Institute.[1]
<figure-inline class="mw-default-size"><figure-inline><figure-inline><figure-inline>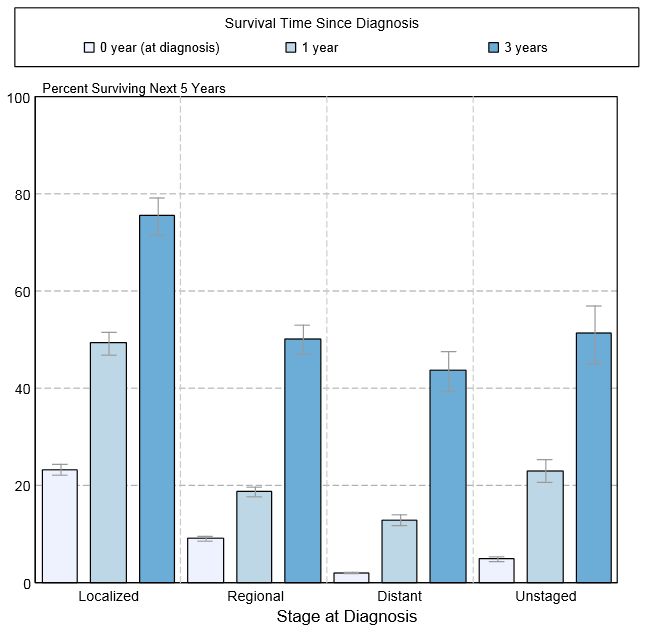
| ORIGIN | DISEASE | DIFFERENTIATION BASED ON INVESTIGATIONS |
|---|---|---|
| Pancreas |
|
|
| Bile duct |
|
|
| Duodenum |
|
|
| Lymphovascular
tissue |
|
|
| Metastasis |
|
|
|
Pancreatic cancer Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Sandbox:Cherry On the Web |
|
American Roentgen Ray Society Images of Sandbox:Cherry |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sudarshana Datta, MD [2]
Obstructive jaundice may be painful due to calculous disease. Patients may be younger.
Stones can be demonstrated on abdominal ultrasound, both in the gallbladder and in the bile duct. However, stones may also be seen in patients with pancreatic cancer. ERCP will clarify the situation by ruling out stricture (seen in pancreatic cancer) and confirming bile duct stones, which can be cleared at the time of intervention.
Treatment Options by Stage
Stages I and II Pancreatic Cancer
Treatment of stage I and stage II pancreatic cancer may include the following:
- Surgery alone.
- Surgery with chemotherapy and radiation therapy.
Stage III Pancreatic Cancer
Treatment of stage III pancreatic cancer may include the following:
- Palliative surgery or stent placement to bypass blocked areas in ducts or the small intestine.
- Chemotherapy with gemcitabine.
Stage IV Pancreatic Cancer
Treatment of stage IV pancreatic cancer may include the following:
- Chemotherapy with gemcitabine with or without erlotinib.
- Palliative treatments for pain, such as nerve blocks, and other supportive care.
- Palliative surgery or stent placement to bypass blocked areas in ducts or the small intestine.
Treatment Options for Recurrent Pancreatic Cancer
Treatment of recurrent pancreatic cancer may include the following:
- Chemotherapy.
- Palliative surgery or stent placement to bypass blocked areas in ducts or the small intestine.
- Palliative radiation therapy.
- Other palliative medical care to reduce symptoms, such as nerve blocks to relieve pain.
prevention
Primary Cessation of cigarette smoking: The risk of pancreatic cancer falls with cessation of cigarette smoking, which is one of the most important modifiable risk factors.[3][4][5][6][7][8][8][9][10] Smoking accounts for the incidence of pancreatic cancer in one-fourth of all cases.[3][11] Nicotine in cigarettes stimulates tumorigenesis, increasing metastasis and resistance to treatment, hence impacting survival in patients.[12] The risk of developing pancreatic cancer becomes almost equivalent to that of a nonsmoker after five years of cessation.[4][13][6][10]
Regular exercise:
Obesity is considered as a potential risk factor for pancreatic cancer.[14]
Regular exercise decreases the risk of pancreatic cancer as compared to people living a sedentary lifestyle.[6][15]
The American Cancer Society (ACS) has issued guidelines for diet and physical activity at individual and community levels. Diet: A healthy balanced diet doesn't exceed 2000 calories daily and includes the following:[7][9][16] plenty of vegetables and fruits- blueberries, spinach, broccoli, tomatoes lean meat from fowl, fish and plant sources like nuts or whole grains monounsaturated fats help control insulin levels in type 2 diabetics[10] Tuna, mackerel, salmon, and sardine are major sources of long-chain omega-3 fatty acids due to anticancer properties
Poor diet: A poor diet includes the presence of the following:[14][4][16] Food preservatives and additives Smoked meat Heavy alcohol use High cholesterol Red meat Low consumption of fruits and vegetables Saturated fatty acids Processed foods high-fat, high-protein diet Chemicals known as heterocyclic amines, nitrates, and heme iron, found in foods, are capable of damaging cells and DNA, influencing cancerogenic processes
Aging: Aging is associated with the development of pancreatic cancer.[13][13][6]
Secondary
Diet: Exocrine pancreatic insufficiency due to pancreatic duct obstruction by the tumor may lead to malabsorption.
Malabsorption in patients presents with anorexia, weight loss, and diarrhea.
Treatment: based on American Cancer Society(ACS) guidelines[17]
Pancreatic enzyme replacement therapy
avoidance of high-protein/high-fat diets
Individualized dietary prescriptions from a registered dietitian
Supplementation with omega-3 fatty acids
Palliative Therapy
- Pain:
- There are various techniques for pain management as palliative therapy in patients with advanced stage of pancreatic cancer:
Pain:
- There are various techniques for pain management as palliative therapy in patients.
- Surgical techniques used to treat pain in advanced pancreatic cancer cases include:
- Endoscopic decompression with stent placement in patients with biliary or pancreatic duct obstruction
- Neurolysis of the celiac ganglia by many approaches:
- Intraoperative
- Transgastric
- Transthoracic
- Transabdominal
Jaundice:
- Obstructive jaundice can present with features of cholangitis:
- Fever and chills
- Nausea, vomiting
- Clay-colored stools
- Dark urine
- Yellowish discoloration of skin
- Pruritus
- Right upper quadrant pain
- Anorexia
- Preferred treatment in patients: Endoscopic decompression with stent placement in patients with biliary obstruction.
- Techniques of biliary decompression:
- Cholecystojejunostomy
- Choledochojejunostomy
- Types of stents:
- Metal- costly, longer lifespan
- Plastic- cheaper, need replacement every three months
Duodenal obstruction
- Preferred treatment:
- ↑ 1.0 1.1 1.2 1.3 Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z,Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975-2011, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2011/, based on November 2013 SEER data submission, posted to the SEER web site, April 2014.
- ↑ Ghaneh P, Costello E, Neoptolemos JP (2007). "Biology and management of pancreatic cancer". Gut. 56 (8): 1134–52. doi:10.1136/gut.2006.103333. PMID 17625148.
- ↑ 3.0 3.1 Bochatay L, Girardin M, Bichard P, Frossard JL (2014). "[Pancreatic cancer in 2014: screening and epidemiology]". Rev Med Suisse (in French). 10 (440): 1582–5. PMID 25276995.
- ↑ 4.0 4.1 4.2 Lowenfels AB, Maisonneuve P (2006). "Epidemiology and risk factors for pancreatic cancer". Best Pract Res Clin Gastroenterol. 20 (2): 197–209. doi:10.1016/j.bpg.2005.10.001. PMID 16549324.
- ↑ Bosetti C, Bertuccio P, Malvezzi M, Levi F, Chatenoud L, Negri E, La Vecchia C (2013). "Cancer mortality in Europe, 2005-2009, and an overview of trends since 1980". Ann. Oncol. 24 (10): 2657–71. doi:10.1093/annonc/mdt301. PMID 23921790.
- ↑ 6.0 6.1 6.2 6.3 Bosetti C, Bertuccio P, Negri E, La Vecchia C, Zeegers MP, Boffetta P (2012). "Pancreatic cancer: overview of descriptive epidemiology". Mol. Carcinog. 51 (1): 3–13. doi:10.1002/mc.20785. PMID 22162227.
- ↑ 7.0 7.1 Hart AR (1999). "Pancreatic cancer: any prospects for prevention?". Postgrad Med J. 75 (887): 521–6. PMC 1741344. PMID 10616684.
- ↑ 8.0 8.1 Vimalachandran D, Ghaneh P, Costello E, Neoptolemos JP (2004). "Genetics and prevention of pancreatic cancer". Cancer Control. 11 (1): 6–14. PMID 14749618.
- ↑ 9.0 9.1 Ghadirian P, Lynch HT, Krewski D (2003). "Epidemiology of pancreatic cancer: an overview". Cancer Detect. Prev. 27 (2): 87–93. PMID 12670518.
- ↑ 10.0 10.1 10.2 Landi S (2009). "Genetic predisposition and environmental risk factors to pancreatic cancer: A review of the literature". Mutat. Res. 681 (2–3): 299–307. doi:10.1016/j.mrrev.2008.12.001. PMID 19150414.
- ↑ Qiu D, Kurosawa M, Lin Y, Inaba Y, Matsuba T, Kikuchi S, Yagyu K, Motohashi Y, Tamakoshi A (2005). "Overview of the epidemiology of pancreatic cancer focusing on the JACC Study". J Epidemiol. 15 Suppl 2: S157–67. PMID 16127228.
- ↑ Toki MI, Syrigos KN, Saif MW (2014). "Risk determination for pancreatic cancer". JOP. 15 (4): 289–91. PMID 25076322.
- ↑ 13.0 13.1 13.2 Li D, Xie K, Wolff R, Abbruzzese JL (2004). "Pancreatic cancer". Lancet. 363 (9414): 1049–57. doi:10.1016/S0140-6736(04)15841-8. PMID 15051286.
- ↑ 14.0 14.1 Bracci PM (2012). "Obesity and pancreatic cancer: overview of epidemiologic evidence and biologic mechanisms". Mol. Carcinog. 51 (1): 53–63. doi:10.1002/mc.20778. PMC 3348117. PMID 22162231.
- ↑ Kollarova H, Azeem K, Tomaskova H, Horakova D, Prochazka V, Martinek A, Shonova O, Sevcikova J, Sevcikova V, Janout V (2014). "Is physical activity a protective factor against pancreatic cancer?". Bratisl Lek Listy. 115 (8): 474–8. PMID 25246281.
- ↑ 16.0 16.1 Kuroczycki-Saniutycz S, Grzeszczuk A, Zwierz ZW, Kołodziejczyk P, Szczesiul J, Zalewska-Szajda B, Ościłowicz K, Waszkiewicz N, Zwierz K, Szajda SD (2017). "Prevention of pancreatic cancer". Contemp Oncol (Pozn). 21 (1): 30–34. doi:10.5114/wo.2016.63043. PMC 5385470. PMID 28435395.
- ↑ Kushi LH, Doyle C, McCullough M, Rock CL, Demark-Wahnefried W, Bandera EV, Gapstur S, Patel AV, Andrews K, Gansler T (2012). "American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity". CA Cancer J Clin. 62 (1): 30–67. doi:10.3322/caac.20140. PMID 22237782.
Treatment:
Psychological therapy: use of Antidepressant medications Caffeine avoidance to decrease anxiety Avoidance of legumes decreases bloating Dietary measures Adjunctive pharmacologic treatment
Dietary Measures: Fiber supplementation -side effect bloating and distension with high fiber diets Individualized dietary recommendations are preferable Flatulence: Polycarbophil compounds (eg, Citrucel, FiberCon)< than psyllium compounds (eg, Metamucil). Judicious water intake is recommended for the constipation-predominant subtype of IBS
Gluten intolerance as patients with gluten/wheat sensitivity may be a subset of those with irritable bowel syndrome. [24] Many patients are interested in dietary manipulation to decrease their symptoms. Several different diets have been proposed. [25] Diets low in FODMAPs (fermentable oligosaccharides, disaccharides, monosaccharides, and polyols) Probiotics are very interesting for treating symptoms, but it is unclear for which patients probiotics are helpful, and in what form, dose, combination, or strain. [27, 28] A meta-analysis concluded that Bifidobacterium infantis may help alleviate some symptoms of irritable bowel syndrome. [29] A systematic review and meta-analysis of 13 articles that assessed the differential expression of intestinal microbiota in 360 patients with this condition compared to 268 healthy controls found downregulation of bacterial colonization of Lactobacillus, Bifidobacterium, and Faecalibacterium prausnitzii in patients with irritable bowel syndrome. [30] Those with the diarrhea-predominant subtype had significantly different expression of Lactobacillus and Bifidobacterium. A different systematic review and meta-analysis evaluated 43 articles on probiotics and showed that probiotics helped relieve pain, bloating, and gas [31] ; however, again, it remains unknown which probiotic is best.
Psychological Therapy Consider psychiatric referral. Previous evidence supported improvement in gastrointestinal (GI) symptoms with successful treatment of psychiatric comorbidities, but studies by Zijdenbos et al and Ford et al indicate that caution should be used when interpreting such data. [33, 34] In a meta-analysis by Zijdenbos et al of 25 randomized trials consisting of single psychological interventions with usual care or mock intervention in patients older than 16 years, the authors found that although cognitive-behavioral therapy and interpersonal psychotherapy were effective immediately after treatment completion, there was no convincing evidence for sustained benefits with any treatment modality. Thus, Zijdenbos et al recommended that future research should focus on current irritable bowel syndrome treatment guidelines and their long-term effects. [33] Ford et al reached similar conclusions regarding the use of psychological interventions in irritable bowel syndrome. The authors concluded that antidepressants are effective in the treatment of irritable bowel syndrome, but although the available data suggest that psychological therapies may be of comparable efficacy, there is less high-quality evidence for the routine use of psychological therapies in patients with IBS. They performed a systematic review and meta-analysis of randomized controlled trials in adults with IBS; however, their selection criteria included trials comparing antidepressants with placebo as well as those comparing psychological therapies with control therapy or usual care. The investigators noted that the quality of studies were generally good for those involving antidepressants but poor for those involving psychological therapy. [34] A Cochrane systematic review determined that antidepressants improved both irritable bowel symptoms and global assessment scores compared with placebo. Selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants were both shown to be effective in subgroup analyses. [4] The 2009 American College of Gastroenterologists (ACG) position statement concluded that psychological interventions, cognitive behavioral therapy, dynamic psychotherapy, and hypnotherapy, are more effective than placebo. Relaxation therapy was no more effective than usual care. In agreement with the above analysis, study quality was described as low. [3] More recent studies suggest targeting the mediating psychological process involved in patients with irritable bowel syndrome, such as illness perceptions, maladaptive coping, and visceral sensitivity. [17] Long-term Monitoring Frequent visits with the clinician enhance the patient-provider relationship, especially in patients who were recently diagnosed with irritable bowel syndrome. Visits can become less frequent as patients are educated and reassured.
Medication SummaryInvestigational use The selection of pharmacologic treatment remains symptom directed. Agents used for the management of symptoms in irritable bowel syndrome (IBS) include anticholinergics, antidiarrheals, tricyclic antidepressants, prokinetics, bulk-forming laxatives, serotonin receptor antagonists, chloride channel activators, and guanylate cyclase C (GC-C) agonists. A Cochrane systematic review found that several antispasmodics, including peppermint oil, pinaverium, trimebutine, and cimetropium/dicyclomine, significantly outperformed placebo at improving irritable bowel syndrome symptom and global assessment scores. [4] The 2009 American College of Gastroenterologists (ACG) position statement on management of irritable bowel syndrome noted that the antidiarrheal agent loperamide effectively reduced stool frequency and improved stool consistency, but it did not relieve pain, bloating, or other global irritable bowel syndrome symptoms. [3] As noted earlier, The 2014 ACG monograph on the management of irritable bowel syndrome and chronic idiopathic constipation found insufficient evidence to recommend prebiotics or synbiotics, or loperamide, in irritable bowel syndrome, and no evidence that polyethylene glycol improved overall symptoms and pain in affected patients. [22] A Spanish expert consensus panel on functional digestive disorders have made evidence-based recommendations on the use of linaclotide, a GC-C receptor agonist, for the management of the constipation-predominant disease (IBS-C) subtype. [35] Their recommendations include continuous (not sporadic) use of linaclotide therapy for moderate to severe IBS-C, patient education regarding the risk of diarrhea and its management options, and the maintenance of linaclotide therapy for potentially long periods on the basis of the lack of tachyphylaxis or potential risks. [35] A total of 1260 patients with IBS without constipation were enrolled in the TARGET 1 and TARGET 2 phase III trials at 179 investigative sites in the United States and Canada. Results showed that treatment with rifaximin (550 mg PO tid for 14 d) provided better symptom relief (eg, bloating, abdominal pain, loose/watery stools) compared with placebo, although the placebo effect was tremendous. Similarly, a 2012 meta-analysis of 5 studies, incorporating 1,803 patients, determined that rifaximin is more effective than placebo for global symptom relief and bloating. Adverse event rates were similar to placebo. [36] Rifaximin is not yet approved by the US Food and Drug Administration for IBS. [37] IBS Agents Class Summary Linaclotide and lubiprostone enhance chloride-rich intestinal fluid secretions without altering sodium and potassium concentrations in the serum. Linaclotide was approved by the FDA in August 2012 to treat chronic idiopathic constipation and irritable bowel syndrome with constipation (IBS-C) in adults. [38] The safety and efficacy of linaclotide in the treatment of IBS-C were evaluated in 2 double-blind, placebo-controlled phase III clinical trials in which linaclotide met all 4 primary endpoints for changes in abdominal pain and constipation in each trial. The trials involved 1,605 patients aged 18-87 years, of which 807 were treated with linaclotide 290 mcg. Both trials showed a significantly higher proportion of responders in the linaclotide group compared with the placebo group. [39, 40] Lubiprostone (Amitiza) View full drug information This agent activates chloride channels on the apical part of the small bowel epithelium. As a result, chloride ions are secreted and sodium and water passively diffuse into the lumen to maintain isotonicity. This medication is FDA approved for use in idiopathic constipation and in irritable bowel syndrome with constipation. Alosetron (Lotronex) View full drug information Alosetron is a 5-HT3 receptor antagonist. This agent controls irritable bowel syndrome symptoms through its potent and selective antagonism of serotonin 5-HT3 receptor type. These receptors are extensively located on the enteric neurons of the GI tract, and stimulation causes hypersensitivity and hyperactivity of the intestine. It is indicated only for women with severe diarrhea-predominant IBS who have: chronic IBS symptoms (generally lasting 6 months or longer), had anatomic or biochemical abnormalities of the GI tract excluded, and have not responded adequately to conventional therapy. Limiting its use to this severely affected population is intended to maximize the benefit-to-risk ratio. The drug was previously removed from the US market but was reintroduced with new restrictions approved by the FDA on June 7, 2002. Restrictions are because of reports of infrequent but serious GI adverse reactions (eg, ischemic colitis, serious complications of constipation), including some that resulted in hospitalization and, rarely, blood transfusion, surgery, or death. In order to prescribe, physicians must be enrolled in the Prescribing Program for Lotronex. Under the new management plan, serious adverse events have been few. [28] Linaclotide (Linzess) View full drug information Guanylate cyclase agonist; activation of guanylate cyclase receptors in the intestinal neurons leads to increased cyclic guanosine monophosphate (cGMP), anion secretion, fluid secretion, and intestinal transit; it appears to work topically rather than systemically; when administered PO, linaclotide activates chloride channels in intestinal epithelial cells to increase intestinal fluid secretion; indicated to treat chronic idiopathic constipation and for IBS-C in adults. Eluxadoline (Viberzi) View full drug information Eluxadoline is a mu opioid receptor agonist. It also is a delta opioid receptor antagonist and a kappa opioid receptor agonist. The multiple opioid activity is designed to treat the symptoms of IBS-D while reducing the incidence of constipation that can occur with unopposed mu opioid receptor agonists. It is indicated for IBS-D in adult men and women. Anticholinergics Class Summary Anticholinergic agents are antispasmodics that inhibit intestinal smooth-muscle depolarization at the muscarinic receptor. These agents help relieve symptoms of intestinal spasms in irritable bowel syndrome. Dicyclomine hydrochloride (Bentyl) View full drug information Dicyclomine blocks the action of acetylcholine at parasympathetic sites in secretory glands, smooth muscle, and CNS. This drug decreases fecal urgency and pain. It is useful in patients with diarrhea-predominant symptoms. Adverse effects are dose dependent. Hyoscyamine sulfate (Levsin) View full drug information Like dicyclomine, hyoscyamine is useful in patients with diarrhea-predominant symptoms and blocks the action of acetylcholine at parasympathetic sites in smooth muscle, secretory glands, and the CNS, which, in turn, has antispasmodic effects. The drug decreases fecal urgency and pain. Antidiarrheals Class Summary These agents are nonabsorbable synthetic opioids. They prolong the GI transit time and decrease secretion via peripheral µ-opioid receptors. They reduce visceral nociception via afferent pathway inhibition. Diphenoxylate hydrochloride 2.5 mg with atropine sulfate 0.025 mg (Lomotil) View full drug information This drug combination consists of 2.5 mg of diphenoxylate, which is a constipating meperidine congener, and 0.025 mg of atropine to discourage abuse. The preparation inhibits excessive GI propulsion and motility, but it may exacerbate constipation. Loperamide (Imodium) View full drug information Loperamide, which is available over the counter, acts on intestinal muscles to inhibit peristalsis and to slow intestinal motility. It prolongs the movement of electrolytes and fluid through bowel and increases the viscosity and loss of fluids and electrolytes. Loperamide improves stool frequency and consistency, reduces abdominal pain and fecal urgency, and may exacerbate constipation. Tricyclic Antidepressants Class Summary Tricyclic antidepressants have both antidepressive and analgesic properties. Agents such as imipramine and amitriptyline are efficacious in treating symptoms of irritable bowel syndrome. The use of tricyclic antidepressants in irritable bowel syndrome is off label. Imipramine (Tofranil) View full drug information Imipramine increases pain threshold in the gut, thereby providing a visceral analgesic effect. It prolongs oral-cecal transit time; reduces abdominal pain, mucorrhea, and stool frequency; and increases global well-being variably. It is effective in irritable bowel syndrome in doses subtherapeutic for antidepressive actions, suggesting an independent mechanism of action in this disorder. Amitriptyline (Elavil) View full drug information Like imipramine, amitriptyline provides a visceral analgesic effect at doses subtherapeutic for antidepressive actions. It also prolongs oral-cecal transit time, reduces abdominal pain, mucorrhea, and stool frequency, and increases global well-being variably. Antibiotics Class Summary Antibiotics may play a role in the treatment of irritable bowel syndrome by preventing the overgrowth of intestinal bacteria. Rifaximin (Xifaxan) View full drug information Rifaximin is a semisynthetic derivative of rifampin and acts by binding to the beta-subunit of bacterial DNA-dependent RNA polymerase, blocking one of the steps in transcription. This results in inhibition of bacterial protein synthesis and consequently inhibits the growth of bacteria. The exact mechanism of action for IBS-D is not known, but it is thought to be related to changes in the bacterial content in the gastrointestinal tract and reduction of gas. It is indicated for IBS-D in adult men and women. Bulk-Forming Laxatives Class Summary These products are made of natural and semi-synthetic hydrophilic polysaccharides and cellulose derivatives that dissolve or swell in the intestinal fluid, forming emollient gels that facilitate the passage of intestinal contents and stimulate peristalsis. As fiber supplements, these products may improve symptoms of constipation and diarrhea, but their use in irritable bowel syndrome is controversial. Methylcellulose (Citrucel) View full drug information This agent promotes bowel evacuation by forming a viscous liquid and promoting peristalsis. Psyllium (Metamucil, Fiberall, Reguloid, Konsyl) View full drug information Like methylcellulose, psyllium promotes bowel evacuation by forming a viscous liquid and promoting peristalsis.