Zollinger-Ellison syndrome pathophysiology: Difference between revisions
No edit summary |
|||
| Line 8: | Line 8: | ||
==Pathogenesis== | ==Pathogenesis== | ||
*Zollinger-Ellison syndrome is a disorder where increased levels of [[gastrin]] are produced, causing the [[stomach]] to produce excess [[hydrochloric acid]]. Often, the cause is a [[tumor]] ([[gastrinoma]]) of the [[duodenum]] or [[pancreas]] producing the hormone [[gastrin]]. Gastrin then causes an excessive production of acid which can lead to [[peptic ulcers]] (in almost 95% of patients).<ref name="wikipedia">wikipedia.2015.https://en.wikipedia.org/wiki/Zollinger%E2%80%93Ellison_syndrome</ref> | *Zollinger-Ellison syndrome is a disorder where increased levels of [[gastrin]] are produced, causing the [[stomach]] to produce excess [[hydrochloric acid]]. Often, the cause is a [[tumor]] ([[gastrinoma]]) of the [[duodenum]] or [[pancreas]] producing the hormone [[gastrin]]. Gastrin then causes an excessive production of acid which can lead to [[peptic ulcers]] (in almost 95% of patients).<ref name="wikipedia">wikipedia.2015.https://en.wikipedia.org/wiki/Zollinger%E2%80%93Ellison_syndrome</ref> | ||
*The gastrinoma tumor cells secrete excessive amounts of gastrin which leads to hyperplasia of the fundic parietal cells and increased basal gastric acid output. The excessive gastric acid output breaches the mucosal defenses of the gastric as well as the duodenal wall, causes ulceration, and inactivates pancreatic digestive enzymes with resultant fat malabsorption and diarrhea. Inhibition of absorption of sodium and water by the small intestine results in a secretory component of the diarrhea. <ref name="pmid28722872">{{cite journal |vauthors=Cingam S, Karanchi H |title= |journal= |volume= |issue= |pages= |year= |pmid=28722872 |doi= |url=}}</ref> | *The gastrinoma tumor cells secrete excessive amounts of gastrin which leads to [[hyperplasia]] of the fundic [[parietal cells]] and increased basal [[gastric acid]] output. The excessive [[gastric acid]] output breaches the mucosal defenses of the gastric as well as the duodenal wall, causes [[ulceration]], and inactivates [[pancreatic]] digestive enzymes with resultant fat [[malabsorption]] and [[diarrhea]]. Inhibition of absorption of sodium and water by the [[small intestine]] results in a secretory component of the [[diarrhea]]. <ref name="pmid28722872">{{cite journal |vauthors=Cingam S, Karanchi H |title= |journal= |volume= |issue= |pages= |year= |pmid=28722872 |doi= |url=}}</ref> | ||
*Gastrin works on stomach [[parietal cell]]s causing them to [[Hydrogen potassium ATPase|secrete]] more [[hydrogen ion]]s into the stomach lumen. In addition, gastrin acts as a trophic factor for [[parietal cells]], causing [[parietal cell]] hyperplasia. Thus, there is an increase in the number of acid secreting cells and each of these cells produces acid at a higher rate. The increase in acidity contributes to the development of [[peptic ulcer]]s in the stomach and [[duodenum]]. High acid levels lead to multiple [[ulcer]]s in the [[stomach]] and [[small bowel]]. | *Gastrin works on stomach [[parietal cell]]s causing them to [[Hydrogen potassium ATPase|secrete]] more [[hydrogen ion]]s into the stomach lumen. In addition, [[gastrin]] acts as a trophic factor for [[parietal cells]], causing [[parietal cell]] [[hyperplasia]]. Thus, there is an increase in the number of acid secreting cells and each of these cells produces acid at a higher rate. The increase in acidity contributes to the development of [[peptic ulcer]]s in the stomach and [[duodenum]]. High acid levels lead to multiple [[ulcer]]s in the [[stomach]] and [[small bowel]]. | ||
*The pathophysiology of ZES is related to the trophic action of gastrin on parietal cells of the gastric antrum and the resulting hypersecretory acid milleu. <ref name="pmid24319020">{{cite journal |vauthors=Epelboym I, Mazeh H |title=Zollinger-Ellison syndrome: classical considerations and current controversies |journal=Oncologist |volume=19 |issue=1 |pages=44–50 |year=2014 |pmid=24319020 |pmc=3903066 |doi=10.1634/theoncologist.2013-0369 |url=}}</ref> | *The pathophysiology of ZES is related to the trophic action of gastrin on parietal cells of the gastric antrum and the resulting hypersecretory acid milleu. <ref name="pmid24319020">{{cite journal |vauthors=Epelboym I, Mazeh H |title=Zollinger-Ellison syndrome: classical considerations and current controversies |journal=Oncologist |volume=19 |issue=1 |pages=44–50 |year=2014 |pmid=24319020 |pmc=3903066 |doi=10.1634/theoncologist.2013-0369 |url=}}</ref> | ||
*An overwhelming majority of patients with this disease consequently develop [[peptic ulcers]], often large and multiple, frequently in distal [[duodenum]] and even proximal [[jejunum]] (an uncommon location for [[ulcers]] resulting from [[Helicobacter pylori]] or the use of [[nonsteroidal anti-inflammatory drugs]]). <ref name="pmid24319020">{{cite journal |vauthors=Epelboym I, Mazeh H |title=Zollinger-Ellison syndrome: classical considerations and current controversies |journal=Oncologist |volume=19 |issue=1 |pages=44–50 |year=2014 |pmid=24319020 |pmc=3903066 |doi=10.1634/theoncologist.2013-0369 |url=}}</ref> | *An overwhelming majority of patients with this disease consequently develop [[peptic ulcers]], often large and multiple, frequently in distal [[duodenum]] and even proximal [[jejunum]] (an uncommon location for [[ulcers]] resulting from [[Helicobacter pylori]] or the use of [[nonsteroidal anti-inflammatory drugs]]). <ref name="pmid24319020">{{cite journal |vauthors=Epelboym I, Mazeh H |title=Zollinger-Ellison syndrome: classical considerations and current controversies |journal=Oncologist |volume=19 |issue=1 |pages=44–50 |year=2014 |pmid=24319020 |pmc=3903066 |doi=10.1634/theoncologist.2013-0369 |url=}}</ref> | ||
| Line 17: | Line 17: | ||
==Associated Conditions== | ==Associated Conditions== | ||
*Multiple endocrine neoplasia type 1 ([[MEN 1]]) | *[[Multiple endocrine neoplasia type 1]] ([[MEN 1]]) | ||
*[[Gastrinoma]] | *[[Gastrinoma]] | ||
*[[Peptic ulcer disease]] | *[[Peptic ulcer disease]] | ||
| Line 25: | Line 25: | ||
==Microscopic Pathology== | ==Microscopic Pathology== | ||
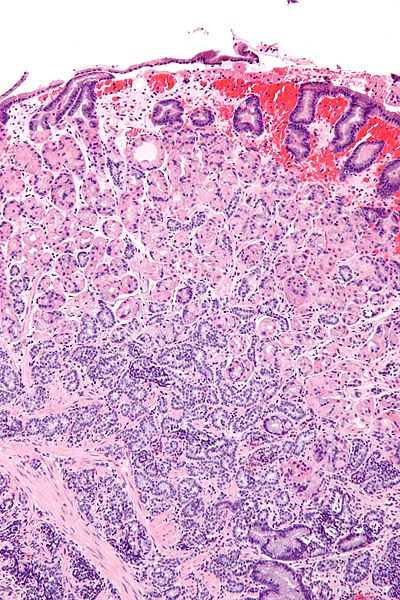
*Histologically, well-differentiated neuroendocrine tumor (NET) has a typical organoid arrangement of cells with nesting, trabecular, or gyriform patterns. <ref name="pmid28722872">{{cite journal |vauthors=Cingam S, Karanchi H |title= |journal= |volume= |issue= |pages= |year= |pmid=28722872 |doi= |url=}}</ref> | *Histologically, well-differentiated [[neuroendocrine tumor]] (NET) has a typical organoid arrangement of cells with nesting, trabecular, or gyriform patterns. <ref name="pmid28722872">{{cite journal |vauthors=Cingam S, Karanchi H |title= |journal= |volume= |issue= |pages= |year= |pmid=28722872 |doi= |url=}}</ref> | ||
*The tumor cells are round with regular bland nuclei and produce large amounts of secretory granules with diffuse immunoexpression of neuroendocrine markers. In contrast, the poorly differentiated NET has atypical, sheet-like, diffuse and irregular nuclei, less cytoplasmic secretory granules, and limited biomarker immunoexpression. <ref name="pmid28722872">{{cite journal |vauthors=Cingam S, Karanchi H |title= |journal= |volume= |issue= |pages= |year= |pmid=28722872 |doi= |url=}}</ref> | *The tumor cells are round with regular bland nuclei and produce large amounts of secretory granules with diffuse immunoexpression of [[neuroendocrine]] markers. In contrast, the poorly differentiated [[neuroendocrine tumor]] (NET) has atypical, sheet-like, diffuse and irregular nuclei, less cytoplasmic secretory granules, and limited biomarker immunoexpression. <ref name="pmid28722872">{{cite journal |vauthors=Cingam S, Karanchi H |title= |journal= |volume= |issue= |pages= |year= |pmid=28722872 |doi= |url=}}</ref> | ||
*An important feature for the diagnosis of neuroendocrine tumors is immunostaining for chromogranin A and synaptophysin. Gastrin immunostaining can be used to differentiate from other neuroendocrine tumors. Gastrinomas express a high density of somatostatin receptors, thus making somatostatin scintigraphy an effective localizing tool. <ref name="pmid28722872">{{cite journal |vauthors=Cingam S, Karanchi H |title= |journal= |volume= |issue= |pages= |year= |pmid=28722872 |doi= |url=}}</ref> | *An important feature for the diagnosis of [[neuroendocrine]] tumors is immunostaining for [[chromogranin A]] and [[synaptophysin]]. Gastrin immunostaining can be used to differentiate from other [[neuroendocrine tumors]]. [[Gastrinoma|Gastrinomas]] express a high density of [[somatostatin]] receptors, thus making [[somatostatin]] [[scintigraphy]] an effective localizing tool. <ref name="pmid28722872">{{cite journal |vauthors=Cingam S, Karanchi H |title= |journal= |volume= |issue= |pages= |year= |pmid=28722872 |doi= |url=}}</ref> | ||
<div align="left"> | <div align="left"> | ||
Revision as of 01:48, 16 August 2017
|
Zollinger-Ellison syndrome Microchapters |
|
Differentiating Zollinger-Ellison syndrome from other Diseases |
|---|
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
Zollinger-Ellison syndrome pathophysiology On the Web |
|
American Roentgen Ray Society Images of Zollinger-Ellison syndrome pathophysiology |
|
Risk calculators and risk factors for Zollinger-Ellison syndrome pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Associate Editor(s)-in-Chief: Aravind Reddy Kothagadi M.B.B.S[2] Mohamad Alkateb, MBBCh [3]
Overview
Development of Zollinger-Ellison syndrome is the result of increased levels of gastrin due to an existing gastrinoma in the duodenum or pancreas.
Pathogenesis
- Zollinger-Ellison syndrome is a disorder where increased levels of gastrin are produced, causing the stomach to produce excess hydrochloric acid. Often, the cause is a tumor (gastrinoma) of the duodenum or pancreas producing the hormone gastrin. Gastrin then causes an excessive production of acid which can lead to peptic ulcers (in almost 95% of patients).[1]
- The gastrinoma tumor cells secrete excessive amounts of gastrin which leads to hyperplasia of the fundic parietal cells and increased basal gastric acid output. The excessive gastric acid output breaches the mucosal defenses of the gastric as well as the duodenal wall, causes ulceration, and inactivates pancreatic digestive enzymes with resultant fat malabsorption and diarrhea. Inhibition of absorption of sodium and water by the small intestine results in a secretory component of the diarrhea. [2]
- Gastrin works on stomach parietal cells causing them to secrete more hydrogen ions into the stomach lumen. In addition, gastrin acts as a trophic factor for parietal cells, causing parietal cell hyperplasia. Thus, there is an increase in the number of acid secreting cells and each of these cells produces acid at a higher rate. The increase in acidity contributes to the development of peptic ulcers in the stomach and duodenum. High acid levels lead to multiple ulcers in the stomach and small bowel.
- The pathophysiology of ZES is related to the trophic action of gastrin on parietal cells of the gastric antrum and the resulting hypersecretory acid milleu. [3]
- An overwhelming majority of patients with this disease consequently develop peptic ulcers, often large and multiple, frequently in distal duodenum and even proximal jejunum (an uncommon location for ulcers resulting from Helicobacter pylori or the use of nonsteroidal anti-inflammatory drugs). [3]
Genetics
- Approximately 80% of the time, the primary causative lesion is thought to arise sporadically; in the remainder of recorded cases, this entity exists as part of MEN-1, an autosomal dominant disorder characterized by tumors of the pituitary, the parathyroid, and the pancreas. [4]
Associated Conditions
Gross Pathology
- Gross pathology presents as enlarged fundic mucosal folds with cerebriform pattern.
Microscopic Pathology
- Histologically, well-differentiated neuroendocrine tumor (NET) has a typical organoid arrangement of cells with nesting, trabecular, or gyriform patterns. [2]
- The tumor cells are round with regular bland nuclei and produce large amounts of secretory granules with diffuse immunoexpression of neuroendocrine markers. In contrast, the poorly differentiated neuroendocrine tumor (NET) has atypical, sheet-like, diffuse and irregular nuclei, less cytoplasmic secretory granules, and limited biomarker immunoexpression. [2]
- An important feature for the diagnosis of neuroendocrine tumors is immunostaining for chromogranin A and synaptophysin. Gastrin immunostaining can be used to differentiate from other neuroendocrine tumors. Gastrinomas express a high density of somatostatin receptors, thus making somatostatin scintigraphy an effective localizing tool. [2]
-
Well-differentiated neuroendocrine tumor of the duodenum.
-
Pancreatic neuroendocrine tumour.
-
Gastric neuroendocrine tumour - high magnification
-
Gastric neuroendocrine tumour - intermed_magnification
-
Gastric neuroendocrine tumour - low magnification
-
Gastrinoma
References
- ↑ wikipedia.2015.https://en.wikipedia.org/wiki/Zollinger%E2%80%93Ellison_syndrome
- ↑ 2.0 2.1 2.2 2.3 Cingam S, Karanchi H. PMID 28722872. Missing or empty
|title=(help) - ↑ 3.0 3.1 Epelboym I, Mazeh H (2014). "Zollinger-Ellison syndrome: classical considerations and current controversies". Oncologist. 19 (1): 44–50. doi:10.1634/theoncologist.2013-0369. PMC 3903066. PMID 24319020.
- ↑ Thakker RV, Newey PJ, Walls GV, Bilezikian J, Dralle H, Ebeling PR; et al. (2012). "Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1)". J Clin Endocrinol Metab. 97 (9): 2990–3011. doi:10.1210/jc.2012-1230. PMID 22723327.