Alzheimer's disease pathophysiology: Difference between revisions
No edit summary |
|||
| Line 44: | Line 44: | ||
}}</ref> | }}</ref> | ||
[[Plaques]] are made of a small [[peptide]] (39 to 43 amino acid residues) called [[beta-amyloid]] (also A-beta or Aβ), a [[protein]] fragment snipped from a larger protein called [[amyloid precursor protein]] (APP). APP is a [[transmembrane protein]]; which means that it sticks through the neuron's membrane; and is believed to help neurons grow, survive and repair themselves after injury.<ref name="pmid16822978">{{cite journal |author=Priller C, Bauer T, Mitteregger G, Krebs B, Kretzschmar HA, Herms J |title=Synapse formation and function is modulated by the amyloid precursor protein |journal=Journal of Neuroscience |volume=26 |issue=27 |pages=7212–7221 |year=2006 |pmid=16822978 |doi=10.1523/JNEUROSCI.1450-06.2006}}</ref><ref name="pmid12927332">{{cite journal |author=Turner PR, O'Connor K, Tate WP, Abraham WC |title=Roles of amyloid precursor protein and its fragments in regulating neural activity, plasticity and memory |journal=Prog. Neurobiology |volume=70 |issue=1 |pages=1–32 |year=2003 |pmid=12927332 |doi=}}</ref> In AD, APP is divided by [[enzymes]] such as gamma-secretase and BACE-1 through a mechanism called [[proteolysis]].<ref name="pmid15787600">{{cite journal |author=Hooper NM |title=Roles of proteolysis and lipid rafts in the processing of the amyloid precursor protein and prion protein |journal=Biochemical Society Transactions |volume=33 |issue=Pt 2 |pages=335–338 |year=2005 |pmid=15787600 |doi=10.1042/BST0330335}}</ref> One of these fragments is [[beta-amyloid]]. Beta-amyloid fragments (amyloid fibrils) outside the cell form clumps that deposit outside neurons in dense formations known as [[senile plaques]].<ref name="pmid15004691">{{cite journal |author=Ohnishi S, Takano K |title=Amyloid fibrils from the viewpoint of protein folding |journal=Cellular Molecular Life Sciences |volume=61 |issue=5 |pages=511–524 |year=2004 |pmid=15004691 |doi=10.1007/s00018-003-3264-8}}</ref><ref name="pmid15184601">{{cite journal |author=Tiraboschi P, Hansen LA, Thal LJ, Corey-Bloom J |title=The importance of neuritic plaques and tangles to the development and evolution of AD |journal=Neurology |volume=62 |issue=11 |pages=1984–1989 |year=2004 |pmid=15184601 |doi=}}</ref> | [[Plaques]] are made of a small [[peptide]] (39 to 43 amino acid residues) called [[beta-amyloid]] (also A-beta or Aβ), a [[protein]] fragment snipped from a larger protein called [[amyloid precursor protein]] (APP). APP is a [[transmembrane protein]]; which means that it sticks through the neuron's membrane; and is believed to help neurons grow, survive and repair themselves after injury.<ref name="pmid16822978">{{cite journal |author=Priller C, Bauer T, Mitteregger G, Krebs B, Kretzschmar HA, Herms J |title=Synapse formation and function is modulated by the amyloid precursor protein |journal=Journal of Neuroscience |volume=26 |issue=27 |pages=7212–7221 |year=2006 |pmid=16822978 |doi=10.1523/JNEUROSCI.1450-06.2006}}</ref><ref name="pmid12927332">{{cite journal |author=Turner PR, O'Connor K, Tate WP, Abraham WC |title=Roles of amyloid precursor protein and its fragments in regulating neural activity, plasticity and memory |journal=Prog. Neurobiology |volume=70 |issue=1 |pages=1–32 |year=2003 |pmid=12927332 |doi=}}</ref> In AD, APP is divided by [[enzymes]] such as gamma-secretase and BACE-1 through a mechanism called [[proteolysis]].<ref name="pmid15787600">{{cite journal |author=Hooper NM |title=Roles of proteolysis and lipid rafts in the processing of the amyloid precursor protein and prion protein |journal=Biochemical Society Transactions |volume=33 |issue=Pt 2 |pages=335–338 |year=2005 |pmid=15787600 |doi=10.1042/BST0330335}}</ref> One of these fragments is [[beta-amyloid]]. Beta-amyloid fragments (amyloid fibrils) outside the cell form clumps that deposit outside neurons in dense formations known as [[senile plaques]].<ref name="pmid15004691">{{cite journal |author=Ohnishi S, Takano K |title=Amyloid fibrils from the viewpoint of protein folding |journal=Cellular Molecular Life Sciences |volume=61 |issue=5 |pages=511–524 |year=2004 |pmid=15004691 |doi=10.1007/s00018-003-3264-8}}</ref><ref name="pmid15184601">{{cite journal |author=Tiraboschi P, Hansen LA, Thal LJ, Corey-Bloom J |title=The importance of neuritic plaques and tangles to the development and evolution of AD |journal=Neurology |volume=62 |issue=11 |pages=1984–1989 |year=2004 |pmid=15184601 |doi=}}</ref> | ||
The amyloid hypothesis is compelling because the gene for the amyloid beta precursor (APP) is located on [[chromosome 21]], and patients with [[trisomy 21]] (Down Syndrome) who thus have an extra [[gene dosage|gene copy]] almost universally exhibit AD-like disorders by 40 years of age.<ref name="pmid16904243">{{cite journal | The amyloid hypothesis is compelling because the gene for the amyloid beta precursor (APP) is located on [[chromosome 21]], and patients with [[trisomy 21]] ([[Down Syndrome]]) who thus have an extra [[gene dosage|gene copy]] almost universally exhibit AD-like disorders by 40 years of age.<ref name="pmid16904243">{{cite journal | ||
|author=Nistor M, Don M, Parekh M, Sarsoza F, Goodus M, Lopez GE, Kawas C, Leverenz J, Doran E, Lott IT, Hill M, Head E | |author=Nistor M, Don M, Parekh M, Sarsoza F, Goodus M, Lopez GE, Kawas C, Leverenz J, Doran E, Lott IT, Hill M, Head E | ||
|title=Alpha- and beta-secretase activity as a function of age and beta-amyloid in Down syndrome and normal brain | |title=Alpha- and beta-secretase activity as a function of age and beta-amyloid in Down syndrome and normal brain | ||
Revision as of 20:06, 7 November 2016
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
|
Alzheimer's disease Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Alzheimer's disease pathophysiology On the Web |
|
American Roentgen Ray Society Images of Alzheimer's disease pathophysiology |
|
Risk calculators and risk factors for Alzheimer's disease pathophysiology |
Overview
Alzheimer's disease develops from the loss of neurons and synaptic connections. There exist at least three major hypotheses that aim to describe the mechanism of how Alzheimer's disease proliferates in the brain.
Pathogenesis

At a macroscopic level, AD is characterized by loss of neurons and synapses in the cerebral cortex and certain subcortical regions. This results in gross atrophy of the affected regions, including degeneration in the temporal lobe and parietal lobe, and parts of the frontal cortex and cingulate gyrus.[1] Three major hypotheses exist to explain the cause of the disease, though other possible explanations also exist.
Cholinergic hypothesis
The oldest major hypothesis is the cholinergic hypothesis, which proposes that AD is caused by reduced synthesis of the neurotransmitter acetylcholine. Most currently available drug therapies in Alzheimer's are based on this theory, although the medications that treat acetylcholine deficiency only affect symptoms of the disease and will neither halt nor reverse the progression of AD.[2] The cholinergic hypothesis has not maintained widespread support in the face of this evidence, although cholinergic effects have been proposed to initiate large-scale aggregation,[3] leading to generalized neuroinflammation.[1] In 1991 the amyloid hypothesis was proposed,[4] while research after 2000 is also centered on tau proteins. The two positions differ insofar as one states that the tau protein abnormalities initiate the disease cascade, while the other states that amyloid beta (Aβ) deposits are the causative factor in the disease.[5] Other changes, including congophilic amyloid angiopathy, oxidative changes, neuronal loss, and inflammation are also associated with Alzheimer's disease.
Amyloid hypothesis
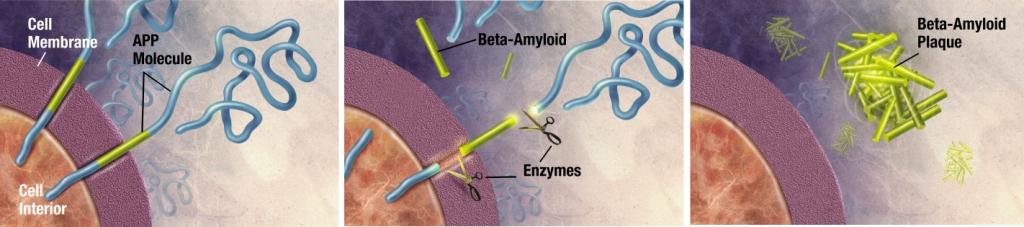
Alzheimer's disease has been identified as a protein misfolding disease, or proteopathy, due to the accumulation of abnormally folded A-beta and tau proteins in the brains of AD patients.[6] The amyloid hypothesis postulates that amyloid beta (Aβ) deposits are the fundamental cause of the disease.[4][5] Plaques are made of a small peptide (39 to 43 amino acid residues) called beta-amyloid (also A-beta or Aβ), a protein fragment snipped from a larger protein called amyloid precursor protein (APP). APP is a transmembrane protein; which means that it sticks through the neuron's membrane; and is believed to help neurons grow, survive and repair themselves after injury.[7][8] In AD, APP is divided by enzymes such as gamma-secretase and BACE-1 through a mechanism called proteolysis.[9] One of these fragments is beta-amyloid. Beta-amyloid fragments (amyloid fibrils) outside the cell form clumps that deposit outside neurons in dense formations known as senile plaques.[10][11] The amyloid hypothesis is compelling because the gene for the amyloid beta precursor (APP) is located on chromosome 21, and patients with trisomy 21 (Down Syndrome) who thus have an extra gene copy almost universally exhibit AD-like disorders by 40 years of age.[12][13] It should be noted further that ApoE4, the major genetic risk factor for AD, leads to excess amyloid build-up in the brain before AD symptoms arise. Thus, Aβ deposition precedes clinical AD.[14] It is known that some types of inherited AD involve only mutations in the APP gene (although this is not the most common type—others involve genes for "pre-senilin" proteins which process APP and may also have still-unknown functions).[15] However, another strong support for the amyloid hypothesis, which looks at Aβ as the common initiating factor for Alzheimer's disease, is that transgenic mice solely expressing a mutant human APP gene develop fibrillar amyloid plaques.[16] If damage from Aβ is the primary initiating cause of AD, the exact mechanism has not been elucidated. The traditional formulation of the amyloid hypothesis points to the cytotoxicity of mature aggregated amyloid fibrils.[17] The most neurotoxic form of amyloid are the soluble oligomers and intermediate amyloids; the severity of cognitive defect in AD correlates with oligomeric level in brain tissue. It is also known that Aβ selectively builds up in the mitochondria of samples from the brains of humans with AD, as well as in mitochondria from transgenic mice with APP genes. In both cases, it inhibits certain mitochondrial enzyme functions and exhibits a similar decrease in glucose utilization in neurons to the one which is a known characteristic of AD. This process may also lead to the formation of damaging reactive oxygen species, calcium influx, and apoptosis. Mechanisms which involve direct damage from Aβ before it forms fibrils and plaques also address the issue that neuronal damage is not correlated as well with plaques, since in this model it is not the plaques themselves which cause the major damage, but rather the precursor Aβ protein directly, via another mechanism.[18] Again, deposition of amyloid plaques does not correlate well with neuron loss.[19]
Tau hypothesis
-
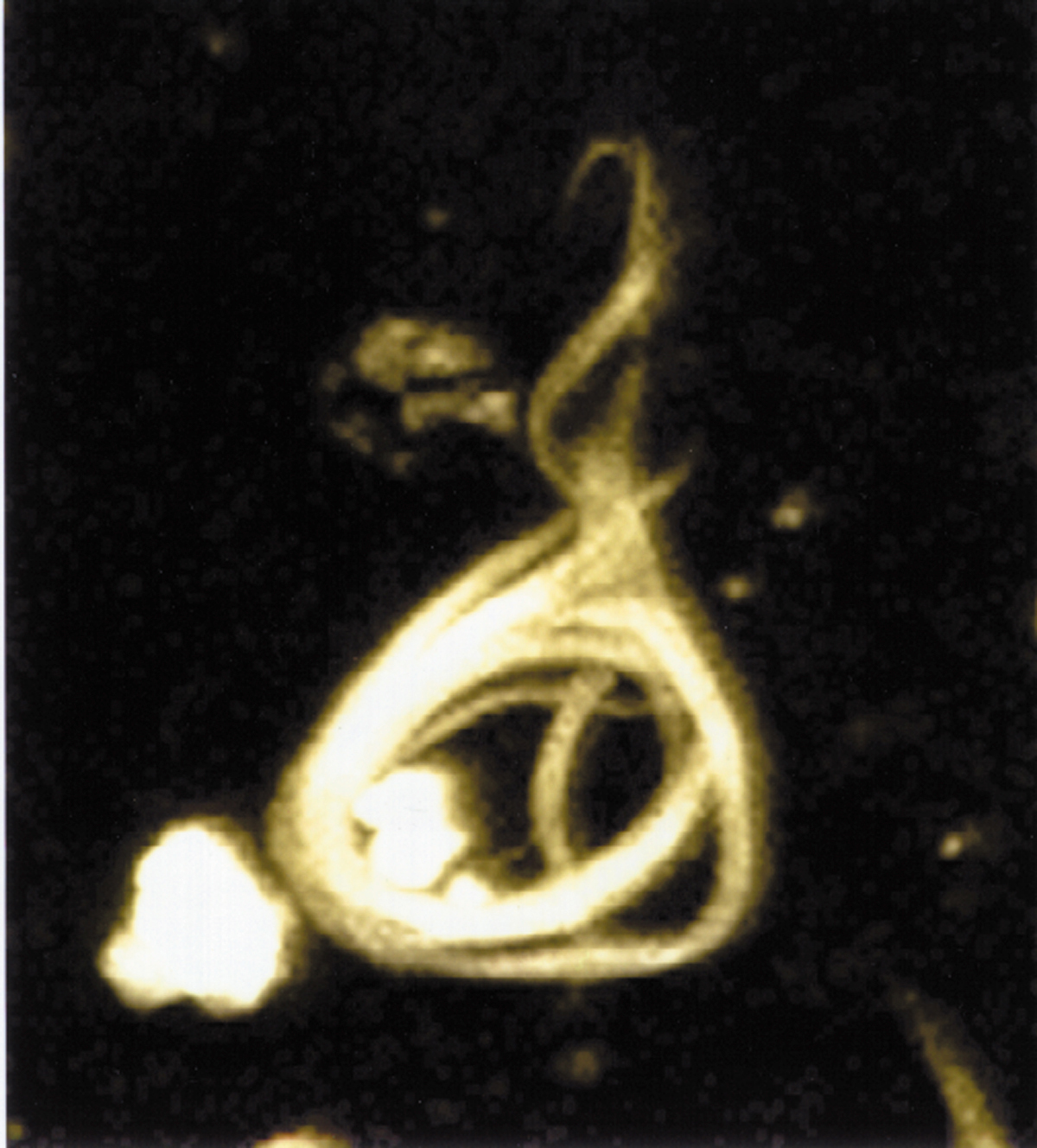
Microscopy image of a neurofibrillary tangle, conformed by hyperphosphorylated tau protein.
-
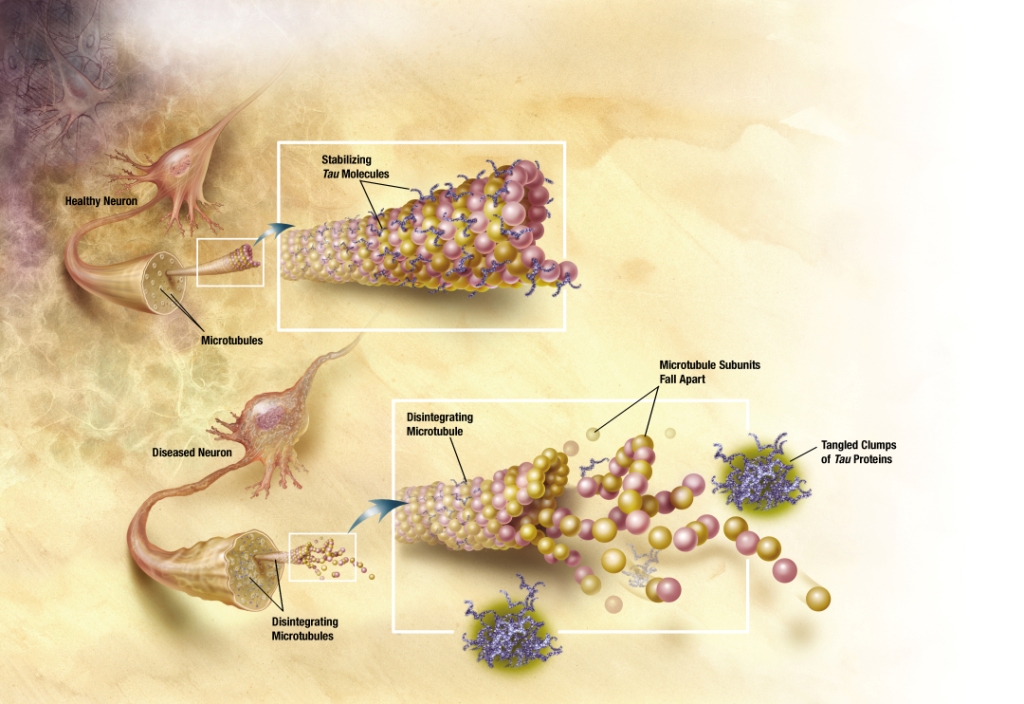
In Alzheimer's disease, changes in tau protein lead to the disintegration of microtubules in brain cells.
The observation that amyloid plaques do not correlate with neuron loss supports the tau hypothesis. The tau hypothesis supports the idea that tau protein abnormalities initiate the disease cascade.[5] AD is also considered a tauopathy due to abnormal aggregation of the tau protein. Healthy neurons have an internal support structure, or cytoskeleton, partly made up of structures called microtubules. These microtubules act like tracks, guiding nutrients and molecules from the body of the cell down to the ends of the axon and back. A special kind of protein, tau, makes the microtubules stable through a process named phosphorylation and is therefore called a microtubule-associated protein.[20] In AD, tau is changed chemically, becoming hyperphosphorylated.
In the tau hypothesis, hyperphosphorylated tau begins to pair with other threads of tau and they become tangled up together inside nerve cell bodies in masses known as neurofibrillary tangles.[21] When this happens, the microtubules disintegrate, collapsing the neuron's transport system. This may result first in malfunctions in communication between neurons and later in the death of the cells.[22] Both amyloid plaques and neurofibrillary tangles are clearly visible by microscopy in AD brains.[11] Plaques are dense, mostly insoluble deposits of amyloid-beta protein and cellular material outside and around neurons. Tangles are insoluble twisted fibers that build up inside the nerve cell. Though many older people develop some plaques and tangles, the brains of AD patients have them to a much greater extent and in different brain locations.[23]
Alternative hypotheses
Recent research supports the previously obscure theory that Herpes simplex virus type 1 plays a role as a possible cause of AD in people carrying the susceptible versions of the apoE gene.[24] As HSV-1 is not a new virus, consideration of additional factors will be needed to explain the increase [2] in the age-adjusted incidence of AD. Various inflammatory processes and inflammatory cytokines may also have a role in the pathology of Alzheimer's disease. However, these are general markers of tissue damage in any disease, and may also be either secondary causes of tissue damage in AD, or else bystander "marker" effects.[25] Other cholinergic effects have also been proposed—for example, the initiation of large-scale aggregation of amyloid,[3] leading to generalized neuroinflammation.[1]
Associated Conditions
References
- ↑ 1.0 1.1 1.2 Wenk GL (2003). "Neuropathologic changes in Alzheimer's disease". Journal of Clinical Psychiatry. 64 Suppl 9: 7–10. PMID 12934968.
- ↑ Walker LC, Rosen RF (2006). "Alzheimer therapeutics-what after the cholinesterase inhibitors?". Age Ageing. 35 (4): 332–335. doi:10.1093/ageing/afl009. PMID 16644763.
- ↑ 3.0 3.1 Shen ZX (2004). "Brain cholinesterases: II. The molecular and cellular basis of Alzheimer's disease". Medical Hypotheses. 63 (2): 308–321. doi:10.1016/j.mehy.2004.02.031. PMID 15236795.
- ↑ 4.0 4.1 Hardy J, Allsop D (1991). "Amyloid deposition as the central event in the aetiology of Alzheimer's disease". Trends Pharmacol. Sci. 12 (10): 383–8. doi:10.1016/0165-6147(91)90609-V. PMID 1763432.
- ↑ 5.0 5.1 5.2 Mudher A, Lovestone S (2002). "Alzheimer's disease-do tauists and baptists finally shake hands?". Trends in Neuroscience. 25 (1): 22–26. doi:10.1016/S0166-2236(00)02031-2. PMID 11801334.
- ↑ Hashimoto M, Rockenstein E, Crews L, Masliah E (2003). "Role of protein aggregation in mitochondrial dysfunction and neurodegeneration in Alzheimer's and Parkinson's diseases". Neuromolecular Medicine. 4 (1–2): 21–36. doi:10.1385/NMM:4:1-2:21. PMID 14528050.
- ↑ Priller C, Bauer T, Mitteregger G, Krebs B, Kretzschmar HA, Herms J (2006). "Synapse formation and function is modulated by the amyloid precursor protein". Journal of Neuroscience. 26 (27): 7212–7221. doi:10.1523/JNEUROSCI.1450-06.2006. PMID 16822978.
- ↑ Turner PR, O'Connor K, Tate WP, Abraham WC (2003). "Roles of amyloid precursor protein and its fragments in regulating neural activity, plasticity and memory". Prog. Neurobiology. 70 (1): 1–32. PMID 12927332.
- ↑ Hooper NM (2005). "Roles of proteolysis and lipid rafts in the processing of the amyloid precursor protein and prion protein". Biochemical Society Transactions. 33 (Pt 2): 335–338. doi:10.1042/BST0330335. PMID 15787600.
- ↑ Ohnishi S, Takano K (2004). "Amyloid fibrils from the viewpoint of protein folding". Cellular Molecular Life Sciences. 61 (5): 511–524. doi:10.1007/s00018-003-3264-8. PMID 15004691.
- ↑ 11.0 11.1 Tiraboschi P, Hansen LA, Thal LJ, Corey-Bloom J (2004). "The importance of neuritic plaques and tangles to the development and evolution of AD". Neurology. 62 (11): 1984–1989. PMID 15184601.
- ↑ Nistor M, Don M, Parekh M, Sarsoza F, Goodus M, Lopez GE, Kawas C, Leverenz J, Doran E, Lott IT, Hill M, Head E (2007). "Alpha- and beta-secretase activity as a function of age and beta-amyloid in Down syndrome and normal brain". Neurobiol. Aging. 28 (10): 1493–506. doi:10.1016/j.neurobiolaging.2006.06.023. PMID 16904243.
- ↑ Lott IT, Head E (2005). "Alzheimer disease and Down syndrome: factors in pathogenesis". Neurobiology of Aging. 26 (3): 383–389. doi:10.1016/j.neurobiolaging.2004.08.005. PMID 15639317.
- ↑ Polvikoski T, Sulkava R, Haltia M, Kainulainen K, Vuorio A, Verkkoniemi A, Niinistö L, Halonen P, Kontula K (1995). "Apolipoprotein E, dementia, and cortical deposition of beta-amyloid protein". New England Journal of Medicine. 333 (19): 1242–1247. doi:10.1056/NEJM199511093331902. PMID 7566000.
- ↑ "Alzheimer disease". US National Library of Medicine. 2008-04-25. Retrieved 2008-05-02.
- ↑ Beta-amyloid precursor protein
- Games D, Adams D, Alessandrini R, Barbour R, Berthelette P, Blackwell C, Carr T, Clemens J, Donaldson T, Gillespie F (1995). "Alzheimer-type neuropathology in transgenic mice overexpressing V717F beta-amyloid precursor protein". Nature. 373 (6514): 523–527. doi:10.1038/373523a0. PMID 7845465.
- Masliah E, Sisk A, Mallory M, Mucke L, Schenk D, Games D (1996). "Comparison of neurodegenerative pathology in transgenic mice overexpressing V717F beta-amyloid precursor protein and Alzheimer's disease". Journal of Neuroscience. 16 (18): 5795–5811. PMID 8795633.
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G (1996). "Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice". Science. 274 (5284): 99–102. doi:10.1126/science.274.5284.99. PMID 8810256.
- ↑ Yankner BA, Duffy LK, Kirschner DA (1990). "Neurotrophic and neurotoxic effects of amyloid beta protein: reversal by tachykinin neuropeptides". Science. 250 (4978): 279–282. doi:10.1126/science.2218531. PMID 2218531.
- ↑ Chen, X, Yan, SD (2006). "Mitochondrial Aβ: A Potential Cause of Metabolic Dysfunction in Alzheimer's Disease". IUBMB Life. 58 (12): 686–694. doi:10.1080/15216540601047767. PMID 17424907.
- ↑ Schmitz C, Rutten BP, Pielen A; et al. (2004). "Hippocampal neuron loss exceeds amyloid plaque load in a transgenic mouse model of Alzheimer's disease". Am. J. Pathol. 164 (4): 1495–1502. PMC 1615337. PMID 15039236. Unknown parameter
|month=ignored (help) - ↑ Hernández F, Avila J (2007). "Tauopathies". Cellular Molecular Life Sciences. 64 (17): 2219–2233. doi:10.1007/s00018-007-7220-x. PMID 17604998.
- ↑ Goedert M, Spillantini MG, Crowther RA (1991). "Tau proteins and neurofibrillary degeneration". Brain Pathology. 1 (4): 279–286. doi:10.1111/j.1750-3639.1991.tb00671.x. PMID 1669718.
- ↑ Chun W, Johnson GV (2007). "The role of tau phosphorylation and cleavage in neuronal cell death". Frontiers of Bioscience. 12: 733–756. PMID 17127334.
- ↑ Bouras C, Hof PR, Giannakopoulos P, Michel JP, Morrison JH (1994). "Regional distribution of neurofibrillary tangles and senile plaques in the cerebral cortex of elderly patients: a quantitative evaluation of a one-year autopsy population from a geriatric hospital". Cerebral Cortex. 4 (2): 138–150. doi:10.1093/cercor/4.2.138. PMID 8038565.
- ↑ Wozniak MA, Mee AP, Itzhaki RF (2009). "Herpes simplex virus type 1 DNA is located within Alzheimer's disease amyloid plaques". J Pathol. 217 (1): 131–8. doi:10.1002/path.2449. PMID 18973185. Unknown parameter
|month=ignored (help) - ↑ Greig NH, Mattson MP, Perry T, Chan SL, Giordano T, Sambamurti K, Rogers JT, Ovadia H, Lahiri DK (2004). "New therapeutic strategies and drug candidates for neurodegenerative diseases: p53 and TNF-alpha inhibitors, and GLP-1 receptor agonists". Ann N Y Acad Sci. 1035 (Dec): 290–315. doi:10.1196/annals.1332.018. PMID 15681814.