Influenza future or investigational therapies: Difference between revisions
| Line 6: | Line 6: | ||
==Future or Investigational Therapies== | ==Future or Investigational Therapies== | ||
[[Peramivir]] is being developed by BioCryst Pharmaceuticals, but has not yet been approved for sale in the United States.<ref> | |||
===Antiviral Medication=== | |||
*[[Peramivir]] is being developed by BioCryst Pharmaceuticals, but has not yet been approved for sale in the United States.<ref> | |||
{{cite web | title = Peramivir Fact Sheet | {{cite web | title = Peramivir Fact Sheet | ||
| publisher = BioCryst Pharmaceuticals Inc | | publisher = BioCryst Pharmaceuticals Inc | ||
| Line 14: | Line 16: | ||
}}</ref> | }}</ref> | ||
===Vaccine Development=== | |||
*One important basic research program is the [[Influenza Genome Sequencing Project]], which is creating a library of influenza sequences; this library should help clarify which factors make one strain more lethal than another, which genes most affect [[immunogenicity]], and how the virus evolves over time.<ref> [http://msc.tigr.org/infl_a_virus/index.shtml Influenza A Virus Genome Project] at The Institute of Genomic Research. Accessed 19 Oct 06</ref> | |||
*Research into new vaccines is particularly important: as current vaccines are slow and expensive to produce and must be reformulated every year. The sequencing of the influenza genome and [[recombinant DNA]] technology may accelerate the generation of new vaccine strains by allowing scientists to substitute new antigens into a previously-developed vaccine strain.<ref>{{cite journal | author = Subbarao K, Katz J | title = Influenza vaccines generated by reverse genetics. | journal = Curr Top Microbiol Immunol | volume = 283 | issue = | pages = 313-42 | year = | id = PMID 15298174}}</ref> | |||
*New technologies are also being developed to grow virus in [[cell culture]]; which promises higher yields, less cost, better quality and surge capacity.<ref>{{cite journal | author = Bardiya N, Bae J | title = Influenza vaccines: recent advances in production technologies. | url=http://www.springerlink.com/content/jdt26gc39v4bwk9q/ | journal = Appl Microbiol Biotechnol | volume = 67 | issue = 3 | pages = 299–305 | year = 2005 | id = PMID 15660212}}</ref> | |||
*The potential [[H5N1]] pandemic has motivated a huge increase in flu research. At least 12 companies and 17 governments are developing pre-pandemic [[influenza vaccine]]s in 28 different clinical trials that, if successful, could turn a deadly pandemic infection into a nondeadly pandemic infection. | |||
*A vaccine that could prevent any illness at all from the not-yet-existing pandemic influenza strain will take at least three months from the virus's emergence until full-scale vaccine production could begin; with vaccine production hoped to increase until one billion doses are produced by one year after the virus is first identified.<ref> [http://www.scidev.net/gateways/index.cfm?fuseaction=readitem&rgwid=2&item=Features&itemid=515&language=1 Science and Development Network] article ''Pandemic flu: fighting an enemy that is yet to exist'' published May 3, 2006.</ref> | |||
*It is estimated that, in a best scenario situation, 750 million doses could be produced each year, whereas it is likely that each individual would need two doses of the vaccine in order to become immuno-competent.<ref>[http://www.phacilitate.co.uk/pages/phaciliate/article_fedson.html phacilitate.co.uk]</ref>. | |||
There are two serious technical problems associated with the development of a vaccine against H5N1. The first problem is | ====Problems with Vacccine Develpment against H5N1==== | ||
*There are two serious technical problems associated with the development of a vaccine against H5N1. | |||
*The first problem is that seasonal influenza vaccines require a single injection of 15 μg haemagluttinin in order to give protection; H5 seems to evoke only a weak immune response and a large multicentre trial found that two injections of 90 µg H5 given 28 days apart provided protection in only 54% of people {{Harv|Treanor|2006}}. | |||
* Even if it is considered that 54% is an acceptable level of protection, the world is currently capable of producing only 900 million doses at a strength of 15 μg (assuming that all production were immediately converted to manufacturing H5 vaccine); if two injections of 90 μg are needed then this capacity drops to only 70 million {{Harv|Poland|2006}}. | |||
*Trials using [[adjuvant]]s such as [[alum]] or MF59 to try and lower the dose of vaccine are urgently needed. | |||
*The second problem is that there are two circulating [[clade]]s of virus, clade 1 is the virus originally isolated in Vietnam, clade 2 is the virus isolated in Indonesia. | |||
*Current vaccine research is focussed on clade 1 viruses, but the clade 2 virus is antigenically distinct and a clade 1 vaccine will probably not protect against a pandemic caused by clade 2 virus. | |||
= | ====Reverse genetics==== | ||
===Reverse genetics=== | |||
[[Image:ReverseGeneticsFlu.jpg|250px|thumb|[[Avian Flu]] vaccine development by [[Reverse genetics]] techniques. '''Courtesy''': [[National Institute of Allergy and Infectious Diseases]]]] | [[Image:ReverseGeneticsFlu.jpg|250px|thumb|[[Avian Flu]] vaccine development by [[Reverse genetics]] techniques. '''Courtesy''': [[National Institute of Allergy and Infectious Diseases]]]] | ||
A technique called [[reverse genetics]] allows scientists to manipulate the [[genome]]s of influenza viruses and to transfer [[gene]]s between viral strains. The technique allows the rapid generation of seed viruses for vaccine candidates that exactly match the anticipated epidemic strain. By removing or modifying certain virulence genes, reverse genetics also can be used to convert highly pathogenic influenza viruses into vaccine candidates that are safer for vaccine manufacturers to handle. | *A technique called [[reverse genetics]] allows scientists to manipulate the [[genome]]s of influenza viruses and to transfer [[gene]]s between viral strains. | ||
*The technique allows the rapid generation of seed viruses for vaccine candidates that exactly match the anticipated epidemic strain. | |||
*By removing or modifying certain virulence genes, reverse genetics also can be used to convert highly pathogenic influenza viruses into vaccine candidates that are safer for vaccine manufacturers to handle. | |||
===Pandemic flu vaccine planning=== | ===Pandemic flu vaccine planning=== | ||
Revision as of 16:24, 29 October 2014
|
Influenza Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Influenza future or investigational therapies On the Web |
|
American Roentgen Ray Society Images of Influenza future or investigational therapies |
|
Risk calculators and risk factors for Influenza future or investigational therapies |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Future or Investigational Therapies
Antiviral Medication
- Peramivir is being developed by BioCryst Pharmaceuticals, but has not yet been approved for sale in the United States.[1]
Vaccine Development
- One important basic research program is the Influenza Genome Sequencing Project, which is creating a library of influenza sequences; this library should help clarify which factors make one strain more lethal than another, which genes most affect immunogenicity, and how the virus evolves over time.[2]
- Research into new vaccines is particularly important: as current vaccines are slow and expensive to produce and must be reformulated every year. The sequencing of the influenza genome and recombinant DNA technology may accelerate the generation of new vaccine strains by allowing scientists to substitute new antigens into a previously-developed vaccine strain.[3]
- New technologies are also being developed to grow virus in cell culture; which promises higher yields, less cost, better quality and surge capacity.[4]
- The potential H5N1 pandemic has motivated a huge increase in flu research. At least 12 companies and 17 governments are developing pre-pandemic influenza vaccines in 28 different clinical trials that, if successful, could turn a deadly pandemic infection into a nondeadly pandemic infection.
- A vaccine that could prevent any illness at all from the not-yet-existing pandemic influenza strain will take at least three months from the virus's emergence until full-scale vaccine production could begin; with vaccine production hoped to increase until one billion doses are produced by one year after the virus is first identified.[5]
- It is estimated that, in a best scenario situation, 750 million doses could be produced each year, whereas it is likely that each individual would need two doses of the vaccine in order to become immuno-competent.[6].
Problems with Vacccine Develpment against H5N1
- There are two serious technical problems associated with the development of a vaccine against H5N1.
- The first problem is that seasonal influenza vaccines require a single injection of 15 μg haemagluttinin in order to give protection; H5 seems to evoke only a weak immune response and a large multicentre trial found that two injections of 90 µg H5 given 28 days apart provided protection in only 54% of people Template:Harv.
- Even if it is considered that 54% is an acceptable level of protection, the world is currently capable of producing only 900 million doses at a strength of 15 μg (assuming that all production were immediately converted to manufacturing H5 vaccine); if two injections of 90 μg are needed then this capacity drops to only 70 million Template:Harv.
- Trials using adjuvants such as alum or MF59 to try and lower the dose of vaccine are urgently needed.
- The second problem is that there are two circulating clades of virus, clade 1 is the virus originally isolated in Vietnam, clade 2 is the virus isolated in Indonesia.
- Current vaccine research is focussed on clade 1 viruses, but the clade 2 virus is antigenically distinct and a clade 1 vaccine will probably not protect against a pandemic caused by clade 2 virus.
Reverse genetics
- A technique called reverse genetics allows scientists to manipulate the genomes of influenza viruses and to transfer genes between viral strains.
- The technique allows the rapid generation of seed viruses for vaccine candidates that exactly match the anticipated epidemic strain.
- By removing or modifying certain virulence genes, reverse genetics also can be used to convert highly pathogenic influenza viruses into vaccine candidates that are safer for vaccine manufacturers to handle.
Pandemic flu vaccine planning
According to the US HHS (United States Department of Health & Human Services) Pandemic Influenza Plan Appendix F: Current HHS Activities last revised on November 8, 2005 [7]:
- Currently, influenza vaccine for the annual, seasonal influenza program comes from four manufacturers. However, only a single manufacturer produces the annual vaccine entirely within the U.S. Thus, if a pandemic occurred and existing U.S.-based influenza vaccine manufacturing capacity was completely diverted to producing a pandemic vaccine, supply would be severely limited. Moreover, because the annual influenza manufacturing process takes place during most of the year, the time and capacity to produce vaccine against potential pandemic viruses for a stockpile, while continuing annual influenza vaccine production, is limited. Since supply will be limited, it is critical for HHS to be able to direct vaccine distribution in accordance with predefined groups (see Appendix D); HHS will ensure the building of capacity and will engage states in a discussion about the purchase and distribution of pandemic influenza vaccine.
- Vaccine production capacity: The protective immune response generated by current influenza vaccines is largely based on viral hemagglutinin (HA) and neuraminidase (NA) antigens in the vaccine. As a consequence, the basis of influenza vaccine manufacturing is growing massive quantities of virus in order to have sufficient amounts of these protein antigens to stimulate immune responses. Influenza vaccines used in the United States and around world are manufactured by growing virus in fertilized hens’ eggs, a commercial process that has been in place for decades. To achieve current vaccine production targets millions of 11-day old fertilized eggs must be available every day of production.
- In the near term, further expansion of these systems will provide additional capacity for the U.S.-based production of both seasonal and pandemic vaccines, however, the surge capacity that will be needed for a pandemic response cannot be met by egg-based vaccine production alone, as it is impractical to develop a system that depends on hundreds of millions of 11-day old specialized eggs on a standby basis. In addition, because a pandemic could result from an avian influenza strain that is lethal to chickens, it is impossible to ensure that eggs will be available to produce vaccine when needed.
- In contrast, cell culture manufacturing technology can be applied to influenza vaccines as they are with most viral vaccines (e.g., polio vaccine, measles-mumps-rubella vaccine, chickenpox vaccine). In this system, viruses are grown in closed systems such as bioreactors containing large numbers of cells in growth media rather than eggs. The surge capacity afforded by cell-based technology is insensitive to seasons and can be adjusted to vaccine demand, as capacity can be increased or decreased by the number of bioreactors or the volume used within a bioreactor. In addition to supporting basic research on cell-based influenza vaccine development, HHS is currently supporting a number of vaccine manufacturers in the advanced development of cell-based influenza vaccines with the goal of developing U.S.-licensed cell-based influenza vaccines produced in the United States.
- Dose-sparing technologies. Current U.S.-licensed vaccines stimulate an immune response based on the quantity of HA (hemagglutinin) antigen included in the dose. Methods to stimulate a strong immune response using less HA antigen are being studied in H5N1 and H9N2 vaccine trials. These include changing the mode of delivery from intramuscular to intradermal and the addition of immune-enhancing adjuvant to the vaccine formulation. Additionally, HHS is soliciting contract proposals from manufacturers of vaccines, adjuvants, and medical devices for the development and licensure of influenza vaccines that will provide dose-sparing alternative strategies.
H5N1 vaccine research
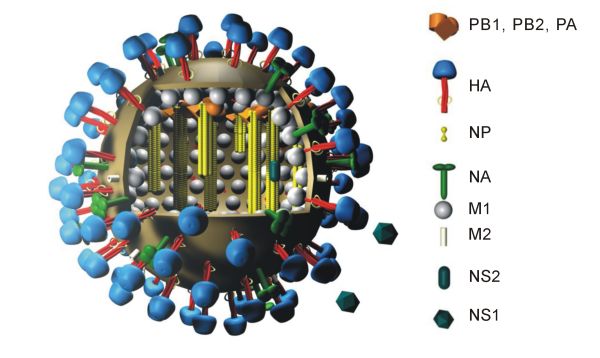
There are several H5N1 vaccines for several of the avian H5N1 varieties. H5N1 continually mutates rendering them, so far for humans, of little use. While there can be some cross-protection against related flu strains, the best protection would be from a vaccine specifically produced for any future pandemic flu virus strain. Dr. Daniel Lucey, co-director of the Biohazardous Threats and Emerging Diseases graduate program at Georgetown University has made this point, "There is no H5N1 pandemic so there can be no pandemic vaccine." However, "pre-pandemic vaccines" have been created; are being refined and tested; and do have some promise both in furthering research and preparedness for the next pandemic. Vaccine manufacturing companies are being encouraged to increase capacity so that if a pandemic vaccine is needed, facilities will be available for rapid production of large amounts of a vaccine specific to a new pandemic strain.
Problems with H5N1 vaccine production include:
- lack of overall production capacity
- lack of surge production capacity (it is impractical to develop a system that depends on hundreds of millions of 11-day old specialized eggs on a standby basis)
- the pandemic H5N1 might be lethal to chickens
Cell culture (cell-based) manufacturing technology can be applied to influenza vaccines as they are with most viral vaccines and thereby solve the problems associated with creating flu vaccines using chicken eggs as is currently done. Researchers at the University of Pittsburgh have had success with a genetically engineered vaccine that took only a month to make and completely protected chickens from the highly pathogenic H5N1 virus. [8]
According to the United States Department of Health & Human Services:
- In addition to supporting basic research on cell-based influenza vaccine development, HHS is currently supporting a number of vaccine manufacturers in the advanced development of cell-based influenza vaccines with the goal of developing U.S.-licensed cell-based influenza vaccines produced in the United States. Dose-sparing technologies. Current U.S.-licensed vaccines stimulate an immune response based on the quantity of HA (hemagglutinin) antigen included in the dose. Methods to stimulate a strong immune response using less HA antigen are being studied in H5N1 and H9N2 vaccine trials. These include changing the mode of delivery from intramuscular to intradermal and the addition of immune-enhancing adjuvant to the vaccine formulation. Additionally, HHS is soliciting contract proposals from manufacturers of vaccines, adjuvants, and medical devices for the development and licensure of influenza vaccines that will provide dose-sparing alternative strategies. [9]
Chiron Corporation is now recertified and under contract with the National Institutes of Health to produce 8,000-10,000 investigational doses of Avian Flu (H5N1) vaccine. Aventis Pasteur is under similar contract.[2] The United States government hopes to obtain enough vaccine in 2006 to treat 4 million people. However, it is unclear whether this vaccine would be effective against a hypothetical mutated strain that would be easily transmitted through human populations, and the shelflife of stockpiled doses has yet to be determined. [10]
The New England Journal of Medicine reported on March 30, 2006 on one of dozens of vaccine studies currently being conducted. The Treanor et al. study was on vaccine produced from the human isolate (A/Vietnam/1203/2004 H5N1) of a virulent clade 1 influenza A (H5N1) virus with the use of a plasmid rescue system, with only the hemagglutinin and neuraminidase genes expressed and administered without adjuvant. "The rest of the genes were derived from an avirulent egg-adapted influenza A/PR/8/34 strain. The hemagglutinin gene was further modified to replace six basic amino acids associated with high pathogenicity in birds at the cleavage site between hemagglutinin 1 and hemagglutinin 2. Immunogenicity was assessed by microneutralization and hemagglutination-inhibition assays with the use of the vaccine virus, although a subgroup of samples were tested with the use of the wild-type influenza A/Vietnam/1203/2004 (H5N1) virus." The results of this study combined with others scheduled to be completed by Spring 2007 is hoped will provide a highly immunogenic vaccine that is cross-protective against heterologous influenza strains. [11]
H5N1 vaccine approval and stockpiling
On April 17, 2007, the first US approval for H5N1 influenza vaccine for humans was given. This vaccine made by Sanofi-Aventis at a plant in Swiftwater, Pennsylvania is not to be sold commercially; instead the US is stockpiling it as an interim measure while better vaccines are being researched. Two injections given 28 days apart gave evidence of providing protection for 45 percent of the people who got the vaccine in a study. "The U.S. Department of Health and Human Services said it had already purchased 13 million doses of the Sanofi vaccine, enough to inoculate 6.5 million people. The vaccine was approved for people age 18 to 64. Studies in other age groups are ongoing. The most common side effects reported were pain at the injection site, headache, general ill feeling and muscle pain, the FDA said."[12]
This April 17, 2007 "approval by the Food and Drug Administration means the vaccine is no longer considered experimental and therefore could be dispensed during a pandemic without requiring each recipient to sign a form giving informed consent. [...] The two injections combined contain 180 micrograms of antigen, the piece of the H5N1 virus designed to spur immunity. By contrast, a conventional flu shot contains 45 micrograms of antigen: 15 micrograms for each of the three strains it protects against."[13]
The vaccine approved on April 17, 2007 "is based on an H5N1 virus isolated from a Vietnamese patient in 2004. Today's approval by the FDA follows a Feb 27 recommendation by an FDA advisory panel, which found that the vaccine was safe and effective. However, some of the panel members had reservations about the immunogenicity of the vaccine, which in data submitted to the panel was somewhat lower than previously reported in a 2006 article in the New England Journal of Medicine. In a clinical trial, two 90-microgram (mcg) doses of the vaccine, administered to 103 healthy adults 28 days apart, generated a protective immune response in 45% of recipients, the FDA noted. (The researchers used a neutralizing antibody titer of 1:40, a fourfold or more increase in antibody titer, to define adequate immune response.) [...] The national stockpile currently contains 13 million doses of the H5N1 vaccine, enough to vaccinate 6.5 million people [...] HHS has said it is moving forward with the development of a "clade 2" H5N1 vaccine, based on viruses that circulated in birds in China and Indonesia in 2003-04 and spread to the Middle East, Europe, and Africa in 2005 and 2006."[14]
Anti-viral drugs
Many nations, as well as the World Health Organization, are working to stockpile anti-viral drugs in preparation for a possible pandemic. Oseltamivir (trade name Tamiflu) is the most commonly sought drug, since it is available in pill form. Zanamivir (trade name Relenza) is also considered for use, but it must be inhaled. Other anti-viral drugs are less likely to be effective against pandemic influenza.
Both Tamiflu and Relenza are in short supply, and production capabilities are limited in the medium term. Some doctors say that co-administration of Tamiflu with probenecid could double supplies. [15]
There also is the potential of viruses to evolve drug resistance. Some H5N1-infected persons treated with oseltamivir have developed resistant strains of that virus.
Peramivir is a pharmaceutical drug used to treat viral infections. Like zanamivir and oseltamivir, peramivir is a neuraminidase inhibitor, acting as a transition-state analogue inhibitor of influenza neuraminidase and thereby preventing new viruses from emerging from infected cells. Experimental data indicate that peramivir may have useful activity against many viruses of interest, including H5N1 (avian bird flu) ,hepatitis B, polio, measles and smallpox. HHS Secretary Mike Leavitt announced on January 4, 2007 that the Department has awarded a $102.6 million, four-year contract to BioCryst Pharmaceuticals, Inc. for advanced development of their influenza antiviral drug, peramivir.[3]
Spanish flu research
One theory is that the virus strain originated at Fort Riley, Kansas, by two genetic mechanisms — genetic drift and antigenic shift — in viruses in poultry and swine which the fort bred for local consumption. But evidence from a recent reconstruction of the virus suggests that it jumped directly from birds to humans, without traveling through swine.[16] On October 5, 2005, researchers announced that the genetic sequence of the 1918 flu strain, a subtype of avian strain H1N1, had been reconstructed using historic tissue samples.[17][18][19] On 18 January 2007, Kobasa et al reported that infected monkeys (Macaca fascicularis) exhibited classic symptoms of the 1918 pandemic and died from a cytokine storm.[20]
References
- ↑ "Peramivir Fact Sheet" (pdf). BioCryst Pharmaceuticals Inc. Retrieved 2007-05-25.
- ↑ Influenza A Virus Genome Project at The Institute of Genomic Research. Accessed 19 Oct 06
- ↑ Subbarao K, Katz J. "Influenza vaccines generated by reverse genetics". Curr Top Microbiol Immunol. 283: 313–42. PMID 15298174.
- ↑ Bardiya N, Bae J (2005). "Influenza vaccines: recent advances in production technologies". Appl Microbiol Biotechnol. 67 (3): 299–305. PMID 15660212.
- ↑ Science and Development Network article Pandemic flu: fighting an enemy that is yet to exist published May 3, 2006.
- ↑ phacilitate.co.uk
- ↑ US HHS (United States Department of Health & Human Services) Pandemic Influenza Plan Appendix F: Current HHS Activities last revised on November 8, 2005
- ↑ Wired News JVI
- ↑ Department of Health & Human Services
- ↑ NPR
- ↑ New England Journal of MedicineVolume 354:1411-1413 - March 30, 2006 - Number 13 - Vaccines against Avian Influenza — A Race against Time
- ↑ Scientific American article U.S. approves first bird flu vaccine for people published April 17, 2007
- ↑ The New York Times article First Vaccine Against Avian Flu Is Approved as Interim Measure published April 18, 2007
- ↑ CIDRAP article FDA approves first H5N1 vaccine published April 17, 2007
- ↑ Nature
- ↑ Sometimes a virus contains both avian adapted genes and human adapted genes. Both the H2N2 and H3N2 pandemic strains contained avian flu virus RNA segments. "While the pandemic human influenza viruses of 1957 (H2N2) and 1968 (H3N2) clearly arose through reassortment between human and avian viruses, the influenza virus causing the 'Spanish flu' in 1918 appears to be entirely derived from an avian source (Belshe 2005)." (from Chapter Two : Avian Influenza by Timm C. Harder and Ortrud Werner, an excellent free on-line Book called Influenza Report 2006 which is a medical textbook that provides a comprehensive overview of epidemic and pandemic influenza.)
- ↑ Special report at Nature News: The 1918 flu virus is resurrected, Published online: 5 October 2005; doi:10.1038/437794a
- ↑ Taubenberger, Jeffery K. (2005). "Characterization of the 1918 influenza virus polymerase genes". Nature. 437: 889–893. doi:10.1038/nature04230. Unknown parameter
|coauthors=ignored (help) - ↑ Also: Tumpey, Terrence M. (2005). "Characterization of the Reconstructed 1918 Spanish Influenza Pandemic Virus". Science. 310: 77–80. doi:10.1126/science.1119392. Unknown parameter
|coauthors=ignored (help) - ↑ Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus Nature. 18 January 2007;445:319