Chlorzoxazone: Difference between revisions
No edit summary |
No edit summary |
||
| Line 17: | Line 17: | ||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Chlorzoxazone in pediatric patients. | |offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Chlorzoxazone in pediatric patients. | ||
|contraindications=* Chlorzoxazone is contraindicated in patients with known intolerance to the drug. | |contraindications=* Chlorzoxazone is contraindicated in patients with known intolerance to the drug. | ||
|warnings=* Serious (including fatal) hepatocellular toxicity has been reported rarely in patients receiving chlorzoxazone. The mechanism is unknown but appears to be idiosyncratic and unpredictable. Factors predisposing patients to this rare event are not known. Patients should be instructed to report early signs and/or symptoms of hepatotoxicity such as fever, rash, anorexia, nausea, vomiting, fatigue, right upper quadrant pain, dark urine, or jaundice. Chlorzoxazone should be discontinued immediately and a physician consulted if any of these signs or symptoms develop. Chlorzoxazone use should also be discontinued if a patient develops abnormal liver enzymes (e.g., AST, ALT, alkaline phosphatase and bilirubin.) | |warnings=* Serious (including fatal) hepatocellular toxicity has been reported rarely in patients receiving chlorzoxazone. The mechanism is unknown but appears to be idiosyncratic and unpredictable. Factors predisposing patients to this rare event are not known. Patients should be instructed to report early signs and/or symptoms of hepatotoxicity such as [[fever]], [[rash]], [[anorexia]], [[nausea]], [[vomiting]], fatigue, right upper quadrant pain, dark urine, or [[jaundice]]. Chlorzoxazone should be discontinued immediately and a physician consulted if any of these signs or symptoms develop. Chlorzoxazone use should also be discontinued if a patient develops abnormal liver enzymes (e.g., AST, ALT, alkaline phosphatase and bilirubin.) | ||
* The concomitant use of alcohol or other central nervous system depressants may have an additive effect. | * The concomitant use of alcohol or other central nervous system depressants may have an additive effect. | ||
=====Usage in Pregnancy===== | =====Usage in Pregnancy===== | ||
* The safe use of chlorzoxazone has not been established with respect to the possible adverse effects upon fetal development. Therefore, it should be used in women of childbearing potential only when, in the judgement of the physician, the potential benefits outweigh the possible risks. | * The safe use of chlorzoxazone has not been established with respect to the possible adverse effects upon fetal development. Therefore, it should be used in women of childbearing potential only when, in the judgement of the physician, the potential benefits outweigh the possible risks. | ||
|clinicalTrials=* Chlorzoxazone containing products are usually well tolerated. It is possible in rare instances that chlorzoxazone may have been associated with gastrointestinal bleeding. [[Drowsiness]], [[dizziness]], [[lightheadedness]], [[malaise]], or over-stimulation may be noted by an occasional patient. Rarely, allergic-type skin rashes, petechiae, or ecchymoses may develop during treatment. [[Angioneurotic edema]] or anaphylactic reactions are extremely rare. There is no evidence that the drug will cause renal damage. Rarely, a patient may note discoloration of the urine resulting from a phenolic metabolite of chlorzoxazone. This finding is of no known clinical significance. | |clinicalTrials=* Chlorzoxazone containing products are usually well tolerated. It is possible in rare instances that chlorzoxazone may have been associated with gastrointestinal bleeding. [[Drowsiness]], [[dizziness]], [[lightheadedness]], [[malaise]], or over-stimulation may be noted by an occasional patient. Rarely, allergic-type skin rashes, [[petechiae]], or [[ecchymoses]] may develop during treatment. [[Angioneurotic edema]] or anaphylactic reactions are extremely rare. There is no evidence that the drug will cause renal damage. Rarely, a patient may note discoloration of the urine resulting from a phenolic metabolite of chlorzoxazone. This finding is of no known clinical significance. | ||
|administration= | |administration= | ||
=====Usual Adult Dosage===== | =====Usual Adult Dosage===== | ||
Revision as of 00:47, 30 July 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Chetan Lokhande, M.B.B.S [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Chlorzoxazone is a skeletal muscle relaxant, spasmolytic that is FDA approved for the treatment of musculoskeletal pain. Common adverse reactions include cardiovascular: lightheadedness, neurologic: dizziness, excitement, paradoxical, somnolence, other: malaise.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Musculoskeletal pain
- 500 to 750 mg orally 3 to 4 times daily
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Chlorzoxazone in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Chlorzoxazone in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Chlorzoxazone FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Chlorzoxazone in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Chlorzoxazone in pediatric patients.
Contraindications
- Chlorzoxazone is contraindicated in patients with known intolerance to the drug.
Warnings
- Serious (including fatal) hepatocellular toxicity has been reported rarely in patients receiving chlorzoxazone. The mechanism is unknown but appears to be idiosyncratic and unpredictable. Factors predisposing patients to this rare event are not known. Patients should be instructed to report early signs and/or symptoms of hepatotoxicity such as fever, rash, anorexia, nausea, vomiting, fatigue, right upper quadrant pain, dark urine, or jaundice. Chlorzoxazone should be discontinued immediately and a physician consulted if any of these signs or symptoms develop. Chlorzoxazone use should also be discontinued if a patient develops abnormal liver enzymes (e.g., AST, ALT, alkaline phosphatase and bilirubin.)
- The concomitant use of alcohol or other central nervous system depressants may have an additive effect.
Usage in Pregnancy
- The safe use of chlorzoxazone has not been established with respect to the possible adverse effects upon fetal development. Therefore, it should be used in women of childbearing potential only when, in the judgement of the physician, the potential benefits outweigh the possible risks.
Adverse Reactions
Clinical Trials Experience
- Chlorzoxazone containing products are usually well tolerated. It is possible in rare instances that chlorzoxazone may have been associated with gastrointestinal bleeding. Drowsiness, dizziness, lightheadedness, malaise, or over-stimulation may be noted by an occasional patient. Rarely, allergic-type skin rashes, petechiae, or ecchymoses may develop during treatment. Angioneurotic edema or anaphylactic reactions are extremely rare. There is no evidence that the drug will cause renal damage. Rarely, a patient may note discoloration of the urine resulting from a phenolic metabolite of chlorzoxazone. This finding is of no known clinical significance.
Postmarketing Experience
There is limited information regarding Chlorzoxazone Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Chlorzoxazone Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Chlorzoxazone in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Chlorzoxazone in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Chlorzoxazone during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Chlorzoxazone in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Chlorzoxazone in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Chlorzoxazone in geriatric settings.
Gender
There is no FDA guidance on the use of Chlorzoxazone with respect to specific gender populations.
Race
There is no FDA guidance on the use of Chlorzoxazone with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Chlorzoxazone in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Chlorzoxazone in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Chlorzoxazone in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Chlorzoxazone in patients who are immunocompromised.
Administration and Monitoring
Administration
Usual Adult Dosage
- One tablet three or four times daily. If adequate response is not obtained with this dose, it may be increased to one and one-half tablets (750 mg) three or four times daily. As improvement occurs dosage can usually be reduced.
Monitoring
There is limited information regarding Chlorzoxazone Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Chlorzoxazone and IV administrations.
Overdosage
Symptoms
- Initially, gastrointestinal disturbances such as nausea, vomiting or diarrhea together with drowsiness, dizziness, lightheadedness or headache may occur. Early in the course there may be malaise or sluggishness followed by marked loss of muscle tone, making voluntary movement impossible. The deep tendon reflexes may be decreased or absent. The sensorium remains intact, and there is no peripheral loss of sensation. Respiratory depression may occur with rapid, irregular respiration and intercostal and substernal retraction. The blood pressure is lowered, but shock has not been observed.
Treatment
- Gastric lavage or induction of emesis should be carried out, followed by administration of activated charcoal. Thereafter, treatment is entirely supportive. If respirations are depressed, oxygen and artificial respiration should be employed and a patent airway assured by use of an oropharyngeal airway or endotracheal tube. Hypotension may be counteracted by use of dextran, plasma, concentrated albumin or a vasopressor agent such as norepinephrine. Cholinergic drugs or analeptic drugs are of no value and should not be used.
Pharmacology
| |
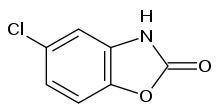
Chlorzoxazone
| |
| Systematic (IUPAC) name | |
| 5-chloro-3H-benzooxazol-2-one | |
| Identifiers | |
| CAS number | |
| ATC code | M03 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 169.565 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | well absorbed |
| Protein binding | 13–18% |
| Metabolism | hepatic |
| Half life | 1.1 hr |
| Excretion | urine (<1%) |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | oral |
Mechanism of Action
- Chlorzoxazone is a centrally-acting agent for painful musculoskeletal conditions. Data available from animal experiments as well as human study indicate that chlorzoxazone acts primarily at the level of the spinal cord and subcortical areas of the brain where it inhibits multisynaptic reflex arcs involved in producing and maintaining skeletal muscle spasm of varied etiology
Structure
- Chlorzoxazone USP is a centrally acting skeletal muscle relaxant, available as tablets of 500 mg for oral administration. Its chemical name is 5-Chloro-2-benzoxazolinone, and its structural formula is:

- Chlorzoxazone USP is a white or practically white, practically odorless, crystalline powder. Chlorzoxazone is slightly soluble in water; sparingly soluble in alcohol, in isopropyl alcohol, and in methanol; soluble in solutions of alkali hydroxides and ammonia.
- Chlorzoxazone tablets contain the inactive ingredients Docusate Sodium, Lactose (hydrous), Magnesium Stearate, Microcrystalline Cellulose, Pregelatinized Starch, Sodium Benzoate, and Sodium Starch Glycolate.
Pharmacodynamics
- The clinical result is a reduction of the skeletal muscle spasm with relief of pain and increased mobility of the involved muscles. Blood levels of chlorzoxazone can be detected in people during the first 30 minutes and peak levels may be reached, in the majority of the subjects, in about 1 to 2 hours after oral administration of chlorzoxazone
Pharmacokinetics
- Chlorzoxazone is rapidly metabolized and is excreted in the urine, primarily in a conjugated form as the glucuronide. Less than one percent of a dose of chlorzoxazone is excreted unchanged in the urine in 24 hours.
Nonclinical Toxicology
There is limited information regarding Chlorzoxazone Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Chlorzoxazone Clinical Studies in the drug label.
How Supplied
- Chlorzoxazone tablets, USP are available as oblong, scored, white tablets debossed with WPI on one side and "39"-"68" on the other side and are packaged in bottles of 100 and 500.
Storage
- Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature].
- Dispense contents with a child-resistant closure (as required) and in a tight container as defined in the USP/NF.
- Keep out of the reach of children.
Images
Drug Images
{{#ask: Page Name::Chlorzoxazone |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Chlorzoxazone |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Chlorzoxazone Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Chlorzoxazone interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Chlorzoxazone Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Chlorzoxazone Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Chlorzoxazone |Label Name=Chlorzoxazone label.png
}}