Ceftriaxone labels and packages: Difference between revisions
Jump to navigation
Jump to search
No edit summary |
No edit summary |
||
| Line 31: | Line 31: | ||
|} | |} | ||
Latest revision as of 18:42, 31 December 2013
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1];Associate Editor(s)-in-Chief: Abdurahman Khalil, M.D. [2]
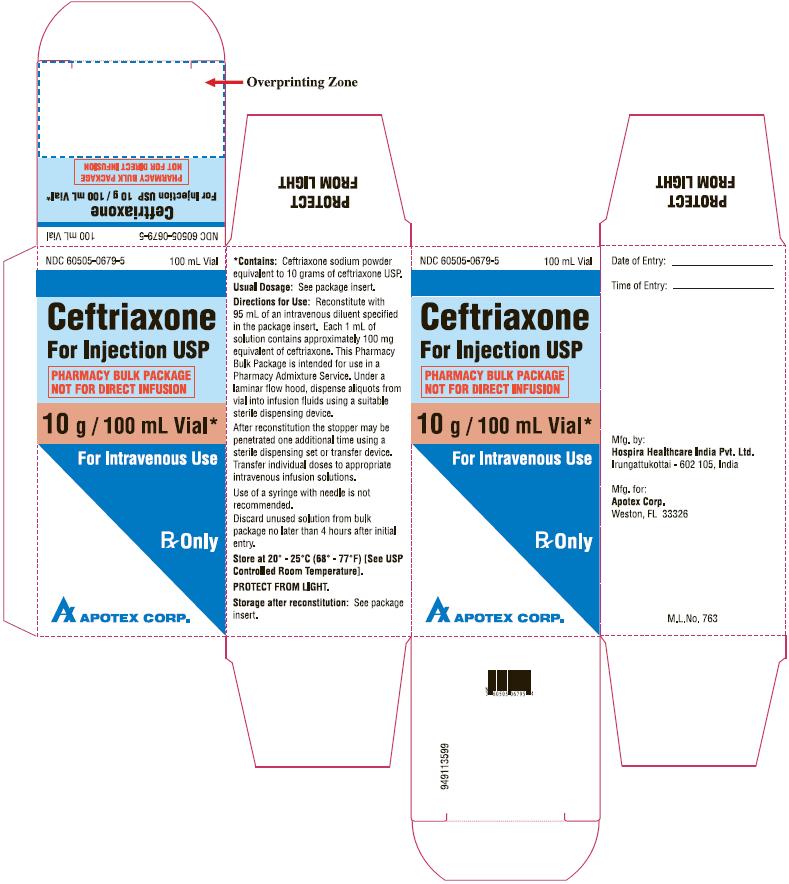
Labels and Packages
Mfg. by:
Hospira Healthcare India Pvt. Ltd.
Irungattukottai - 602 105, India
Mfg. for:
Apotex Corp.
Weston, FL 33326
DATE OF REVISION: JUNE 2011
948026010
949113599-Apotex
 |
 |
References
http://www.accessdata.fda.gov/drugsatfda_docs/label/2005/050796lbl.pdf
|400px|thumb