Secondary amyloidosis pathophysiology: Difference between revisions
No edit summary |
|||
| (One intermediate revision by the same user not shown) | |||
| Line 42: | Line 42: | ||
| colspan="2" style="background:#4479BA; color: #FFFFFF;" align="center" + |'''Associated Conditions''' | | colspan="2" style="background:#4479BA; color: #FFFFFF;" align="center" + |'''Associated Conditions''' | ||
|- | |- | ||
! style="background: #DCDCDC; padding: 5px; text-align: center;" |'''Conditions''' | |||
|'''Examples''' | |'''Examples''' | ||
|- | |- | ||
| Line 134: | Line 134: | ||
|- | |- | ||
|} | |} | ||
== Gross Pathology == | == Gross Pathology == | ||
On [[gross pathology]], the organs affected by amyloidosis can be characterized by the following features: | On [[gross pathology]], the organs affected by amyloidosis can be characterized by the following features: | ||
Latest revision as of 15:40, 10 February 2020
|
Secondary amyloidosis Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Secondary amyloidosis pathophysiology On the Web |
|
American Roentgen Ray Society Images of Secondary amyloidosis pathophysiology |
|
Risk calculators and risk factors for Secondary amyloidosis pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sahar Memar Montazerin, M.D.[2] Shaghayegh Habibi, M.D.[3] Sabawoon Mirwais, M.B.B.S, M.D.[4]
Overview
AA amyloid is an abnormal insoluble extracellular protein derived from the spontaneous aggregation of fibrils composed of misfolded proteins, called amyloid precursors. In secondary amyloidosis, the amyloid precursor is SAA, an acute phase reactant, encoded by SAA 1&2 genes. In secondary amyloidosis, intermediate products of SAA metabolism tend to aggregate and form protofilaments that accumulate within extracellular space and form the insoluble amyloid deposits. Secondary amyloidosis is associated with chronic inflammation (such as tuberculosis or rheumatoid arthritis).
Pathophysiology
- AA amyloid is an abnormal insoluble extracellular protein derived from the spontaneous aggregation of fibrils composed of misfolded proteins, called amyloid precursors.[1][2]
- In secondary amyloidosis, the amyloid precursor is SAA, an acute phase reactant, encoded by SAA 1&2 genes.
- Normally, SAA increased during the inflammatory process and then taken up by macrophages and degraded within lysosomal compartments.
- In patients with secondary amyloidosis, intermediate products of SAA metabolism tend to aggregate and form protofilaments.
- These protofilaments with other protein materials accumulate within extracellular space and form the insoluble amyloid deposits.
- Amyloid deposition can disrupt tissue structure of involved organ and consequently leads to organ failure.[3]
- This algorithm explains the simplified pathogenesis of secondary amyloidosis.
| Infection/Inflammation | |||||||||||||||||||||||
| Increased production of IL-1/IL-6/TNF-α | |||||||||||||||||||||||
| Upregulation of hepatic serum amyloid A production | |||||||||||||||||||||||
| SAA production uptake by macrophages | |||||||||||||||||||||||
| C-terminal cleavage of SAA | |||||||||||||||||||||||
| β-sheet configuration of SAA | |||||||||||||||||||||||
| Fibril deposition in extracellular space | |||||||||||||||||||||||
| Binding of glycosaminoglycan, serum amyloid P, and lipid components | |||||||||||||||||||||||
| Resistant to proteolysis | |||||||||||||||||||||||
| The above algorithm is adopted from International Journal of Nephrology and Renovascular Disease[4] |
|---|
Associated Conditions
- Conditions associated with AA amyloidosis include:[5][6][7]
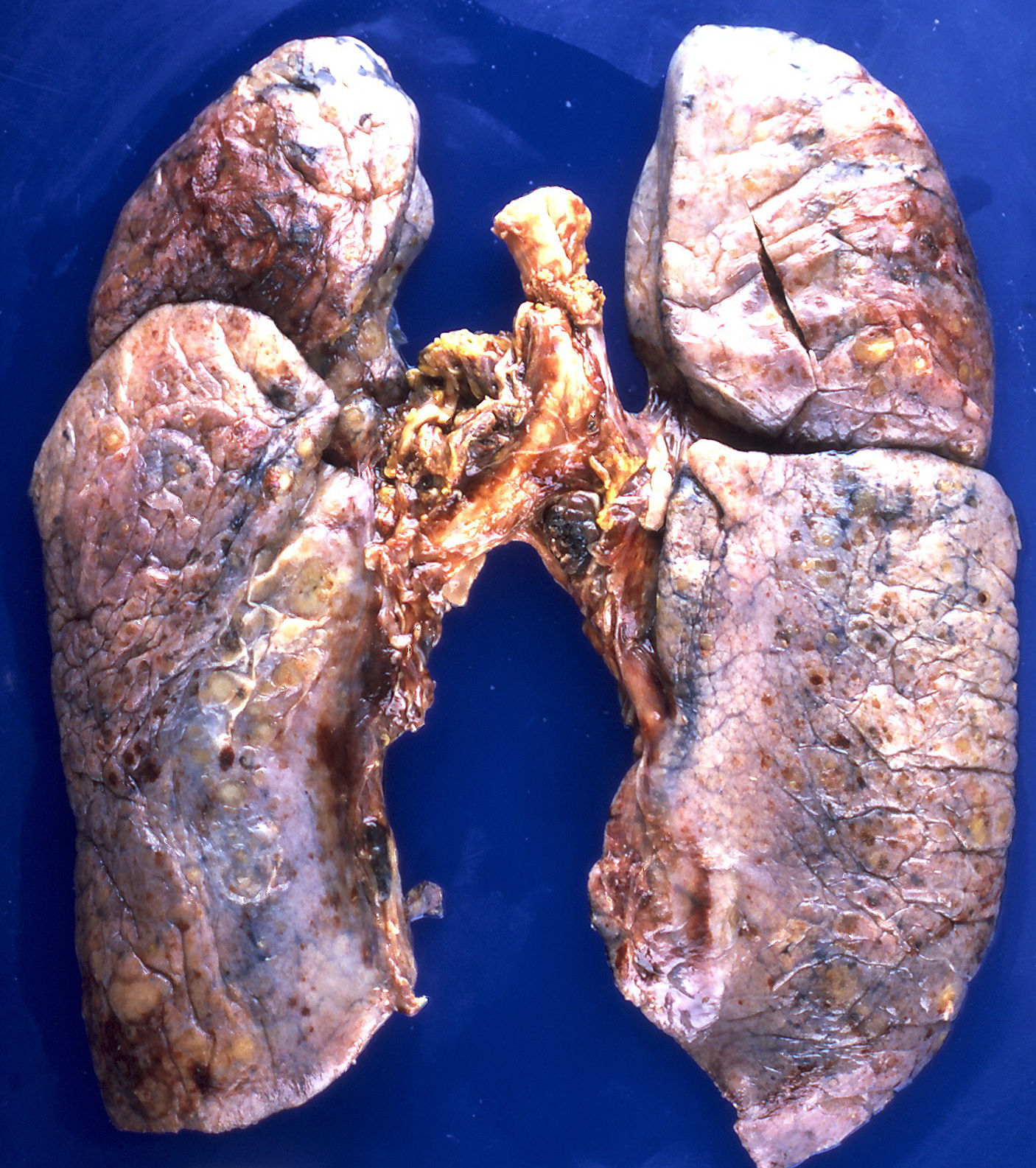
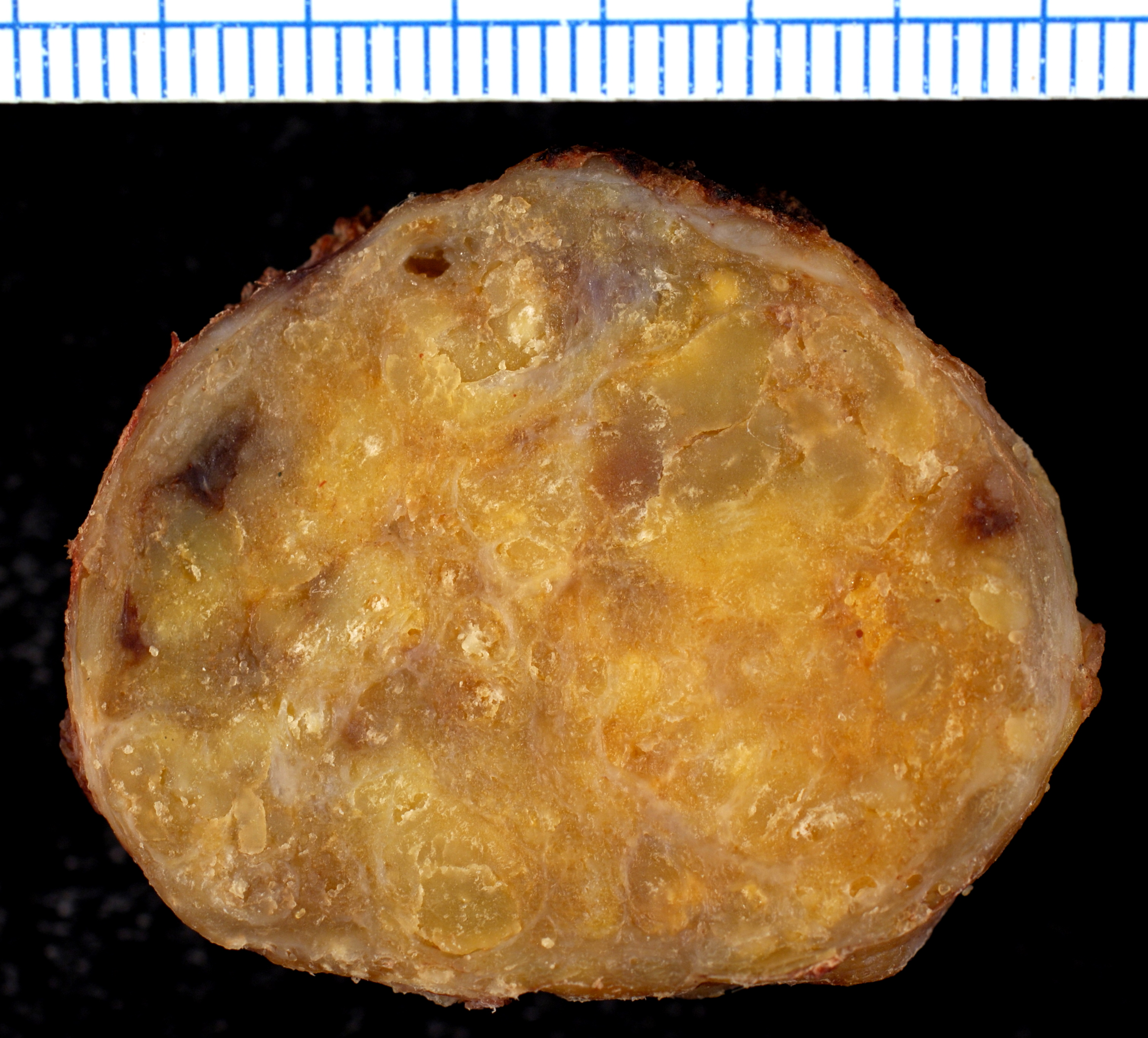
Gross Pathology
On gross pathology, the organs affected by amyloidosis can be characterized by the following features:
- Porcelain like or waxy appearance
- Enlargement
Images

Microscopic Pathology
On microscopic histopathological analysis, aa amyloidosis is characterized by:[11][12]
- Green birefringence under polarized light after Congo red staining (appears red under normal light)
- Linear non-branching fibrils (indefinite length with an approximately same diameter)
- Distinct X-ray diffraction pattern consistent with Pauling's model of a cross-beta fibril
On electron microscopy, amyloid fibrils have the following characteristics:[13]
- 10 to 15 nm diameter
- Straight, rigid, and nonbranching
- Composed of twisted protofilaments
Images



References
- ↑ Jayaraman, Shobini; Gantz, Donald L.; Haupt, Christian; Gursky, Olga (2017). "Serum amyloid A forms stable oligomers that disrupt vesicles at lysosomal pH and contribute to the pathogenesis of reactive amyloidosis". Proceedings of the National Academy of Sciences. 114 (32): E6507–E6515. doi:10.1073/pnas.1707120114. ISSN 0027-8424.
- ↑ Kisilevsky, Robert; Raimondi, Sara; Bellotti, Vittorio (2016). "Historical and Current Concepts of Fibrillogenesis and In vivo Amyloidogenesis: Implications of Amyloid Tissue Targeting". Frontiers in Molecular Biosciences. 3. doi:10.3389/fmolb.2016.00017. ISSN 2296-889X.
- ↑ Wechalekar AD, Gillmore JD, Hawkins PN (June 2016). "Systemic amyloidosis". Lancet. 387 (10038): 2641–2654. doi:10.1016/S0140-6736(15)01274-X. PMID 26719234.
- ↑ Rumjon A, Coats T, Javaid MM (2012). "Review of eprodisate for the treatment of renal disease in AA amyloidosis". Int J Nephrol Renovasc Dis. 5: 37–43. doi:10.2147/IJNRD.S19165. PMC 3304340. PMID 22427728.
- ↑ Blank, Norbert; Hegenbart, Ute; Dietrich, Sascha; Brune, Maik; Beimler, Jörg; Röcken, Christoph; Müller-Tidow, Carsten; Lorenz, Hanns-Martin; Schönland, Stefan O. (2018). "Obesity is a significant susceptibility factor for idiopathic AA amyloidosis". Amyloid. 25 (1): 37–45. doi:10.1080/13506129.2018.1429391. ISSN 1350-6129.
- ↑ van der Hilst, J. C. H.; Yamada, T.; Op den Camp, H. J. M.; van der Meer, J. W. M.; Drenth, J. P. H.; Simon, A. (2008). "Increased susceptibility of serum amyloid A 1.1 to degradation by MMP-1: potential explanation for higher risk of type AA amyloidosis". Rheumatology. 47 (11): 1651–1654. doi:10.1093/rheumatology/ken371. ISSN 1462-0324.
- ↑ Papa, Riccardo; Doglio, Matteo; Lachmann, Helen J.; Ozen, Seza; Frenkel, Joost; Simon, Anna; Neven, Bénédicte; Kuemmerle-Deschner, Jasmin; Ozgodan, Huri; Caorsi, Roberta; Federici, Silvia; Finetti, Martina; Trachana, Maria; Brunner, Jurgen; Bezrodnik, Liliana; Pinedo Gago, Mari Carmen; Maggio, Maria Cristina; Tsitsami, Elena; Al Suwairi, Wafaa; Espada, Graciela; Shcherbina, Anna; Aksu, Guzide; Ruperto, Nicolino; Martini, Alberto; Ceccherini, Isabella; Gattorno, Marco (2017). "A web-based collection of genotype-phenotype associations in hereditary recurrent fevers from the Eurofever registry". Orphanet Journal of Rare Diseases. 12 (1). doi:10.1186/s13023-017-0720-3. ISSN 1750-1172.
- ↑ By Yale Rosen from USA - Amyloidosis, CC BY-SA 2.0, https://commons.wikimedia.org/w/index.php?curid=31127928
- ↑ By Ed Uthman, MD - https://www.flickr.com/photos/euthman/377537238/, CC BY-SA 2.0, https://commons.wikimedia.org/w/index.php?curid=1629764
- ↑ By Ed Uthman, MD - https://www.flickr.com/photos/euthman/377538012/, CC BY-SA 2.0, https://commons.wikimedia.org/w/index.php?curid=1629740
- ↑
- ↑ Röcken C, Shakespeare A (February 2002). "Pathology, diagnosis and pathogenesis of AA amyloidosis". Virchows Arch. 440 (2): 111–122. doi:10.1007/s00428-001-0582-9. PMID 11964039.
- ↑ Close, William; Neumann, Matthias; Schmidt, Andreas; Hora, Manuel; Annamalai, Karthikeyan; Schmidt, Matthias; Reif, Bernd; Schmidt, Volker; Grigorieff, Nikolaus; Fändrich, Marcus (2018). "Physical basis of amyloid fibril polymorphism". Nature Communications. 9 (1). doi:10.1038/s41467-018-03164-5. ISSN 2041-1723.
- ↑ By Michael Feldman, MD, PhDUniversity of Pennsylvania School of Medicine - http://www.healcentral.org/healapp/showMetadata?metadataId=38717, CC BY 2.0, https://commons.wikimedia.org/w/index.php?curid=870218
- ↑ By Ed Uthman, MD - https://www.flickr.com/photos/euthman/377559787/, CC BY-SA 2.0, https://commons.wikimedia.org/w/index.php?curid=1629716
- ↑ By Ed Uthman, MD - https://www.flickr.com/photos/euthman/377559955/, CC BY-SA 2.0, https://commons.wikimedia.org/w/index.php?curid=1629705