Lymphoid leukemia: Difference between revisions
No edit summary |
Nima Nasiri (talk | contribs) |
||
| (112 intermediate revisions by 2 users not shown) | |||
| Line 23: | Line 23: | ||
==Overview== | ==Overview== | ||
'''Lymphoid leukemia''' is a [[monoclonal]] disorder which is a result of clonal proliferation and progressive accumulation of functionally incompetent [[lymphocytes]] in circulation, [[Bone marrow|bone]] [[Bone marrow|marrow]], [[spleen]], [[lymphoid]] tissues. [[Lymphocytic]] leukemia is more frequent than non-lymphocytic and other [[Myeloproliferative disease|myeloproliferative]] diseases. The chronic form ([[CLL]]) affects older adult, onset is insidious. [[Acute lymphoblastic leukemia]] (ALL) is more common in children, peak incidence around 2 to 3 years of age. The identification of cytogenetic abnormalities is highly relevant for the prognosis of [[ALL]]. Patients may have findings associated with [[anemia]], [[neutropenia]], and/or [[thrombocytopenia]] due to [[bone marrow]] involvement. The [[white blood cell]] count may be decreased, normal, or markedly elevated. Symptoms can include [[fatigue]], [[infections]], or easy/spontaneous bruising or bleeding. [[Arthralgias]] and constitutional symptoms (eg, [[fever]], [[night sweats]], unintentional [[weight loss]]) are often present but are generally mild. [[Hepatomegaly]], [[splenomegaly]], and/or [[lymphadenopathy]] can be seen as well. [[Central nervous system]] (CNS) involvement may present as cranial [[Neuropathy|neuropathies]] or [[meningeal]] symptoms. [[Lymphoblasts]] can have different surface molecules called [[cluster of differentiation]] (CD) which can be detected by [[flow cytometry]]. | |||
==Classification== | ==Classification== | ||
**B lymphoblastic leukemia/lymphoma with recurrent genetic abnormalities: | === [[ALL]] classification === | ||
There are two types of classifications for acute lymphoblastic leukemia : | |||
'''[[World Health Organization]]''' ('''WHO''') and '''French-American-British''' ('''FAB''')<ref name="pmid26980727">{{cite journal |vauthors=Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz AD, Jaffe ES |title=The 2016 revision of the World Health Organization classification of lymphoid neoplasms |journal=Blood |volume=127 |issue=20 |pages=2375–90 |date=May 2016 |pmid=26980727 |pmc=4874220 |doi=10.1182/blood-2016-01-643569 |url=}}</ref><ref name="pmid10643532">{{cite journal |vauthors=Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J, Lister TA, Bloomfield CD |title=The World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues. Report of the Clinical Advisory Committee meeting, Airlie House, Virginia, November, 1997 |journal=Ann. Oncol. |volume=10 |issue=12 |pages=1419–32 |date=December 1999 |pmid=10643532 |doi= |url=}}</ref><ref name="pmid1">{{cite journal |vauthors=Makar AB, McMartin KE, Palese M, Tephly TR, Schmoldt A, Benthe HF, Haberland G, Anke H, Spector LB |title=Formate assay in body fluids: application in methanol poisoning |journal=Biochem Med |volume=13 |issue=2 |pages=117–26 |date=June 1975 |pmid=1 |pmc=5922622 |doi= |url=}}</ref><ref name="pmid27161658">{{cite journal |vauthors=Wang Y, Miller S, Roulston D, Bixby D, Shao L |title=Genome-Wide Single-Nucleotide Polymorphism Array Analysis Improves Prognostication of Acute Lymphoblastic Leukemia/Lymphoma |journal=J Mol Diagn |volume=18 |issue=4 |pages=595–603 |date=July 2016 |pmid=27161658 |doi=10.1016/j.jmoldx.2016.03.004 |url=}}</ref> | |||
'''[[WHO]] classification of acute lymphoblastic leukemia''' | |||
*B lymphoblastic [[leukemia]]/[[lymphoma]]: | |||
**B lymphoblastic leukemia/[[lymphoma]], Not otherwise specified | |||
**B lymphoblastic leukemia/lymphoma with recurrent [[genetic]] abnormalities: | |||
**B lymphoblastic leukemia/lymphoma with [[t(9;22)]],[[Bcr-abl|BCR-ABL]]<ref name="pmid26514535">{{cite journal |vauthors=Goud TM, Al Salmani KK, Al Harasi SM, Al Musalhi M, Wasifuddin SM, Rajab A |title=Importance of FISH combined with Morphology, Immunophenotype and Cytogenetic Analysis of Childhood/ Adult Acute Lymphoblastic Leukemia in Omani Patients |journal=Asian Pac. J. Cancer Prev. |volume=16 |issue=16 |pages=7343–50 |date=2015 |pmid=26514535 |doi= |url=}}</ref> | |||
**B lymphoblastic leukemia/lymphoma t(11q23); [[MLL]] rearrangement<ref name="pmid16478880">{{cite journal |vauthors=Nagayama J, Tomizawa D, Koh K, Nagatoshi Y, Hotta N, Kishimoto T, Takahashi Y, Kuno T, Sugita K, Sato T, Kato K, Ogawa A, Nakahata T, Mizutani S, Horibe K, Ishii E |title=Infants with acute lymphoblastic leukemia and a germline MLL gene are highly curable with use of chemotherapy alone: results from the Japan Infant Leukemia Study Group |journal=Blood |volume=107 |issue=12 |pages=4663–5 |date=June 2006 |pmid=16478880 |doi=10.1182/blood-2005-11-4728 |url=}}</ref> | |||
**B lymphoblastic leukemia/lymphoma with t(12;21)<ref name="pmid19594616">{{cite journal |vauthors=Peter A, Heiden T, Taube T, Körner G, Seeger K |title=Interphase FISH on TEL/AML1 positive acute lymphoblastic leukemia relapses--analysis of clinical relevance of additional TEL and AML1 copy number changes |journal=Eur. J. Haematol. |volume=83 |issue=5 |pages=420–32 |date=November 2009 |pmid=19594616 |doi=10.1111/j.1600-0609.2009.01315.x |url=}}</ref> | |||
**B lymphoblastic leukemia/lymphoma with hyperdiploidy | |||
**B lymphoblastic leukemia/lymphoma with hypodiploidy<ref name="pmid10512165">{{cite journal |vauthors=Greipp PR, Trendle MC, Leong T, Oken MM, Kay NE, Van Ness B, Kyle RA |title=Is flow cytometric DNA content hypodiploidy prognostic in multiple myeloma? |journal=Leuk. Lymphoma |volume=35 |issue=1-2 |pages=83–9 |date=September 1999 |pmid=10512165 |doi=10.3109/10428199909145707 |url=}}</ref> | |||
**B lymphoblastic leukemia/lymphoma t(5;14) | |||
**B lymphoblastic leukemia/lymphoma t(1;19) | |||
*T lymphoblastic leukemia/lymphoma: | |||
'''FAB classification of acute lymphoblastic leukemia (for historical purposes):'''<ref name="pmid28052366">{{cite journal |vauthors=Canaani J, Beohou E, Labopin M, Socié G, Huynh A, Volin L, Cornelissen J, Milpied N, Gedde-Dahl T, Deconinck E, Fegueux N, Blaise D, Mohty M, Nagler A |title=Impact of FAB classification on predicting outcome in acute myeloid leukemia, not otherwise specified, patients undergoing allogeneic stem cell transplantation in CR1: An analysis of 1690 patients from the acute leukemia working party of EBMT |journal=Am. J. Hematol. |volume=92 |issue=4 |pages=344–350 |date=April 2017 |pmid=28052366 |doi=10.1002/ajh.24640 |url=}}</ref> | |||
*[[ALL]]-L1: Small cells with homogeneous nuclear [[chromatin]], a regular nuclear shape, small or no [[nucleoli]], scanty [[cytoplasm]], and mild to moderate [[basophilia]] | |||
*[[ALL]]-L2: Large, [[heterogeneous]] cells with variable nuclear [[chromatin]], an irregular nuclear shape, one or more [[nucleoli]], a variable amount of [[cytoplasm]], and basophilia | |||
*[[ALL]]-L3: Large, [[homogeneous]] cells with fine, stippled [[chromatin]]; regular [[nuclei]]; prominent [[nucleoli]]; and abundant, deeply [[basophilic]] [[cytoplasm]]. The most distinguishing feature is prominent [[cytoplasmic]] vacuolation. | |||
=== [[CLL]] classification === | |||
*There are two staging systems in order to classify [[CLL]]: | |||
**'''Rai staging system''' (this is used more often in the United States, it is based on [[lymphocytosis]]) | |||
**'''Stage Characteristics:''' | |||
***'''Low Risk (Stage 0):''' Abnormal increase in the number of [[lymphocytes]] in the blood and marrow. | |||
***'''Intermediate Risk (Stages I & II):''' Abnormal increase in the number of [[lymphocytes]] in the blood and the marrow, enlarged [[lymph]] nodes or abnormal increase in the number of [[lymphocytes]] in the circulating blood and the marrow, enlarged [[spleen]] and/or [[liver]]. | |||
***'''High Risk (Stages III & IV):''' Abnormal increase in the number of [[lymphocytes]] in the circulating blood and the marrow, [[anemia]] ([[hemoglobin]] <11g/dL) or abnormal increase in the number of [[lymphocytes]] in the circulating blood and the marrow [[thrombocytopenia]] ([[platelets]] counts <100,000/uL). | |||
**'''[[Binet staging system]]''' (this is more often used in Europe) <ref name="pmid28091403">{{cite journal |vauthors=Li H, Yi SH, Xiong WJ, Liu HM, Lyu R, Wang TY, Liu W, Zhong SZ, Yu Z, Zou DH, Xu Y, An G, Li ZJ, Qiu LG |title=Chronic Lymphocytic Leukemia Prognostic Index: A New Integrated Scoring System to Predict the Time to First Treatment in Chinese Patients with Chronic Lymphocytic Leukemia |journal=Chin. Med. J. |volume=130 |issue=2 |pages=135–142 |date=January 2017 |pmid=28091403 |pmc=5282668 |doi=10.4103/0366-6999.197978 |url=}}</ref> | |||
***In the Binet staging system, [[CLL]] is classified by the number of affected lymphoid tissue groups (neck lymph nodes, groin [[lymph nodes]], underarm [[lymph nodes]], [[spleen]], and [[liver]]) and by whether or not the patient has [[anemia]] (too few red blood cells) or [[thrombocytopenia]] (too few blood platelets).<ref name="pmid28120419">{{cite journal |vauthors=Delgado J, Doubek M, Baumann T, Kotaskova J, Molica S, Mozas P, Rivas-Delgado A, Morabito F, Pospisilova S, Montserrat E |title=Chronic lymphocytic leukemia: A prognostic model comprising only two biomarkers (IGHV mutational status and FISH cytogenetics) separates patients with different outcome and simplifies the CLL-IPI |journal=Am. J. Hematol. |volume=92 |issue=4 |pages=375–380 |date=April 2017 |pmid=28120419 |doi=10.1002/ajh.24660 |url=}}</ref> | |||
****'''Binet stage A:''' Fewer than 3 areas of [[lymphoid]] tissue are enlarged, with no anemia or [[thrombocytopenia]]. | |||
****'''Binet stage B:''' 3 or more areas of [[lymphoid]] tissue are enlarged, with no [[anemia]] or [[thrombocytopenia]]. | |||
****'''Binet stage C:''' [[Anemia]] and/or [[thrombocytopenia]] are present. Any number of lymphoid tissue areas may be enlarged. | |||
==Pathophysiology== | ==Pathophysiology== | ||
===Physiology=== | |||
*All [[lymphocytes]] have a common lymphoid [[progenitor cell]] origin known as a [[Lymphoblasts|lymphoblast]], the formation of lymphocytes is known as [[lymphopoiesis]]. | |||
*[[B cells]] mature into [[B lymphocytes]] in the [[bone marrow]], while [[T cells]] migrate to and mature in the [[thymus]]. Following maturation, the [[lymphocytes]] enter the circulation and peripheral [[lymphoid]] organs, where they survey for invading [[pathogens]] and [[cancer]] cells. | |||
*<nowiki/>The lymphocytes involved in [[adaptive immunity]] (B and T cells) differentiate further after exposure to an [[antigen]], which occurs in the [[lymph nodes]] during [[antigen]] presentation from the [[Dendritic cells|dendritic cell]]<nowiki/>[[Dendritic cells|s.]]<ref name="pmid30039426">{{cite journal |vauthors=van de Loosdrecht AA, van Wetering S, Santegoets SJAM, Singh SK, Eeltink CM, den Hartog Y, Koppes M, Kaspers J, Ossenkoppele GJ, Kruisbeek AM, de Gruijl TD |title=A novel allogeneic off-the-shelf dendritic cell vaccine for post-remission treatment of elderly patients with acute myeloid leukemia |journal=Cancer Immunol. Immunother. |volume=67 |issue=10 |pages=1505–1518 |date=October 2018 |pmid=30039426 |pmc=6182404 |doi=10.1007/s00262-018-2198-9 |url=}}</ref> | |||
*The fully differentiated [[B cell|B]] and [[T-Cell|T]] cells are specific to the presented antigen and work to defend the body against [[pathogens]] associated with that [[antigen]].<ref name="pmid28411378">{{cite journal |vauthors=Khoury HJ, Collins RH, Blum W, Stiff PS, Elias L, Lebkowski JS, Reddy A, Nishimoto KP, Sen D, Wirth ED, Case CC, DiPersio JF |title=Immune responses and long-term disease recurrence status after telomerase-based dendritic cell immunotherapy in patients with acute myeloid leukemia |journal=Cancer |volume=123 |issue=16 |pages=3061–3072 |date=August 2017 |pmid=28411378 |doi=10.1002/cncr.30696 |url=}}</ref><ref name="pmid17067945">{{cite journal |vauthors=Cesta MF |title=Normal structure, function, and histology of mucosa-associated lymphoid tissue |journal=Toxicol Pathol |volume=34 |issue=5 |pages=599–608 |date=2006 |pmid=17067945 |doi=10.1080/01926230600865531 |url=}}</ref> | |||
*[[Lymphoid]] tissues are subdivided int<nowiki/>o <nowiki/>primary and secondary [[lymphoid organs]]:<ref name="pmid20154733">{{cite journal |vauthors=Bertrand JY, Chi NC, Santoso B, Teng S, Stainier DY, Traver D |title=Haematopoietic stem cells derive directly from aortic endothelium during development |journal=Nature |volume=464 |issue=7285 |pages=108–11 |date=March 2010 |pmid=20154733 |pmc=2858358 |doi=10.1038/nature08738 |url=}}</ref><ref name="pmid17318232">{{cite journal |vauthors=Dorshkind K, Montecino-Rodriguez E |title=Fetal B-cell lymphopoiesis and the emergence of B-1-cell potential |journal=Nat. Rev. Immunol. |volume=7 |issue=3 |pages=213–9 |date=March 2007 |pmid=17318232 |doi=10.1038/nri2019 |url=}}</ref><ref name="pmid23940259">{{cite journal |vauthors=Vossenkämper A, Blair PA, Safinia N, Fraser LD, Das L, Sanders TJ, Stagg AJ, Sanderson JD, Taylor K, Chang F, Choong LM, D'Cruz DP, Macdonald TT, Lombardi G, Spencer J |title=A role for gut-associated lymphoid tissue in shaping the human B cell repertoire |journal=J. Exp. Med. |volume=210 |issue=9 |pages=1665–74 |date=August 2013 |pmid=23940259 |pmc=3754866 |doi=10.1084/jem.20122465 |url=}}</ref> | |||
**The primary [[lymphoid]] tissues responsible for the initial generation of B and T lymphocytes are the [[bone marrow]] and [[thymus]], respectively. | |||
**Secondary lymphoid tissues include [[lymph nodes]], spleen, [[tonsils]], gut-associated lymphoid tissue ([[GALT]]), bronchus-associated lymphoid tissue ( [[BALT]]). | |||
***Within these lymphoid organs, B and T [[lymphocytes]] tend to home to different domains, leading to the segregation of B and [[T cells]]. | |||
***Specifically, [[B cells]] mainly localize to follicles, whereas [[T cells]] mainly localize to interfollicular areas. | |||
***Non-lymphoid cells (eg, [[dendritic cells]], [[monocytes]]/[[Macrophage|macrophages]], [[endothelial cells]], and follicular [[dendritic cells]]) contribute to the formation of these distinct microenvironments, within which specific cell-cell interactions occur that are required for the generation of cellular and [[Humoral immune response|humoral immune responses]]. | |||
===Pathogenesis=== | |||
*It is understood that lymphoid [[leukemia]] is a result of overproduction of cells which is caused by either activation or inactivation of genes.<ref name="pmid28399885">{{cite journal |vauthors=Quijada-Álamo M, Hernández-Sánchez M, Robledo C, Hernández-Sánchez JM, Benito R, Montaño A, Rodríguez-Vicente AE, Quwaider D, Martín AÁ, García-Álvarez M, Vidal-Manceñido MJ, Ferrer-Garrido G, Delgado-Beltrán MP, Galende J, Rodríguez JN, Martín-Núñez G, Alonso JM, García de Coca A, Queizán JA, Sierra M, Aguilar C, Kohlmann A, Hernández JÁ, González M, Hernández-Rivas JM |title=Next-generation sequencing and FISH studies reveal the appearance of gene mutations and chromosomal abnormalities in hematopoietic progenitors in chronic lymphocytic leukemia |journal=J Hematol Oncol |volume=10 |issue=1 |pages=83 |date=April 2017 |pmid=28399885 |pmc=5387353 |doi=10.1186/s13045-017-0450-y |url=}}</ref><ref name="pmid10577857">{{cite journal |vauthors=Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J, Lister TA, Bloomfield CD |title=World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting-Airlie House, Virginia, November 1997 |journal=J. Clin. Oncol. |volume=17 |issue=12 |pages=3835–49 |date=December 1999 |pmid=10577857 |doi=10.1200/JCO.1999.17.12.3835 |url=}}</ref><ref name="pmid23987584">{{cite journal |vauthors=Zhang S, Kipps TJ |title=The pathogenesis of chronic lymphocytic leukemia |journal=Annu Rev Pathol |volume=9 |issue= |pages=103–18 |date=2014 |pmid=23987584 |doi=10.1146/annurev-pathol-020712-163955 |url=}}</ref><ref name="pmid30045301">{{cite journal |vauthors=Ge H, Wu X, Shen J, Chen J, Chen Y, Zhang Y |title=A case report of extranodal NK/T-cell lymphoma in patient with chronic lymphocytic leukemia |journal=Medicine (Baltimore) |volume=97 |issue=30 |pages=e11619 |date=July 2018 |pmid=30045301 |pmc=6078727 |doi=10.1097/MD.0000000000011619 |url=}}</ref> | |||
*Several factors, such as chromosomal [[translocations]] as well as genetic or epigenetic alterations, are involved in [[leukemogenesis]]. | |||
*Abnormal [[methylation]] of [[DNA]] and [[histone]] modifications are important mechanisms in [[tumor suppressor]] silencing, contributing to leukemogenesis along with genetic alterations.<ref name="pmid19718392">{{cite journal |vauthors=Kondo Y |title=Epigenetic cross-talk between DNA methylation and histone modifications in human cancers |journal=Yonsei Med. J. |volume=50 |issue=4 |pages=455–63 |date=August 2009 |pmid=19718392 |pmc=2730606 |doi=10.3349/ymj.2009.50.4.455 |url=}}</ref> | |||
*The activation of [[oncogenes]] involves [[genetic]] changes to cellular [[Proto-oncogene|proto-oncogenes]]. | |||
*Three genetic mechanisms activate [[oncogenes]] in human [[Neoplasm|neoplasms]], these mechanisms result in either an alteration of [[proto-oncogene]] structure or an increase in proto-oncogene expression: | |||
**[[Mutation]] | |||
**[[Gene amplification]] | |||
**[[Chromosome]] rearrangements | |||
===Genetics=== | |||
*Activation or/and inactivation of [[genes]] plays an important role in the pathogenesis and prognosis of lymphoid leukemia. | |||
*Epigenetic and [[genetic]] alterations are two mechanisms in [[leukemia]]. | |||
*Abnormal [[methylation]] of [[DNA]] and [[histone]] modifications are important mechanisms in [[tumor suppressor]] silencing, contributing to leukemogenesis along with genetic alterations. | |||
*Epigenetic mechanisms are the most prevalent inactivation ones in lymphoid leukemia and involve the [[genes]] implicated in several [[cellular]] mechanisms, including [[gene expression]] and [[transcription]], cell-cycle regulation and [[apoptosis]].<ref name="pmid29125235">{{cite journal |vauthors=Gladkikh AA, Potashnikova DM, Tatarskiy V, Yastrebova M, Khamidullina A, Barteneva N, Vorobjev I |title=Comparison of the mRNA expression profile of B-cell receptor components in normal CD5-high B-lymphocytes and chronic lymphocytic leukemia: a key role of ZAP70 |journal=Cancer Med |volume=6 |issue=12 |pages=2984–2997 |date=December 2017 |pmid=29125235 |pmc=5727315 |doi=10.1002/cam4.1257 |url=}}</ref> | |||
** | |||
* | |||
===Associated Conditions=== | |||
Conditions associated with [[lymphoid leukemia]] include:<ref name="pmid29526963">{{cite journal |vauthors=Ito Y, Makita S, Maeshima AM, Hatta S, Suzuki T, Yuda S, Fukuhara S, Munakata W, Suzuki T, Maruyama D, Izutsu K |title=Paraneoplastic Pemphigus Associated with B-cell Chronic Lymphocytic Leukemia Treated with Ibrutinib and Rituximab |journal=Intern. Med. |volume=57 |issue=16 |pages=2395–2398 |date=August 2018 |pmid=29526963 |pmc=6148183 |doi=10.2169/internalmedicine.0578-17 |url=}}</ref> | |||
*[[Anemia]] | |||
*Other cancers such as [[melanoma]] | |||
*[[Lymphadenopathy|Lymph node enlargement]] | |||
*Low grade [[fever]] | |||
*Unexplained [[weight loss]] | |||
*[[Night sweats]] | |||
*[[Splenomegaly|Enlarged spleen]] or [[Hepatomegaly|liver]] | |||
*Infections of the [[skin]], [[lungs]], [[kidneys]] or other sites, as result of low [[immunoglobulin]] levels and decreased [[neutrophil]] counts. | |||
*[[Fatigue]] | |||
*[[Shortness of breath]] during normal physical activity | |||
== Differentiating Lymphoid Leukemia == | |||
{| class="wikitable" | |||
|+ | |||
!Characteristics | |||
!ALL | |||
!CLL | |||
|- | |||
|Microscopy | |||
|[[File:Acute leukemia-ALL.jpg|100x|thumb|center|ALL, hand-mirror cells. [https://commons.wikimedia.org/wiki/File:Acute_leukemia-ALL.jpg Source: wikimedia, transferred from wikipedia]]] | |||
|[[File:Chronic lymphocytic leukemia.jpg|400px|thumb|center|CLL , Smudge cells. [http://en.wikipedia.org en.wikipedia Source: Mary Ann Thompson]]] | |||
|- | |||
|Age of onset | |||
|Children (age < 10 years old) | |||
|Adult onset | |||
|- | |||
|Etiology | |||
|Chromosomal aberration resulting in abnormal [[transcription factors]] that affect development of B and [[T cells]] | |||
|[[Chromosome|Chromosomal]] deletion or possible [[somatic mutation]] of naive [[B cells]] | |||
|- | |||
|Morphology | |||
|Scanty, [[basophilic]] [[cytoplasm]] sometimes with a single long projection (‘hand-mirror cell’), condensed [[chromatin]], small [[nucleoli]]<ref name="pmid3455677">{{cite journal |vauthors=Mazur EM, Wittels EG, Schiffman FJ, South K, Horner RJ |title=Hand mirror cell lymphoid leukemia in adults. A distinct clinicopathologic syndrome. Case report and literature review |journal=Cancer |volume=57 |issue=1 |pages=92–9 |date=January 1986 |pmid=3455677 |doi= |url=}}</ref><ref name="pmid22207681">{{cite journal |vauthors=Tang G, Zuo Z, Thomas DA, Lin P, Liu D, Hu Y, Kantarjian HM, Bueso-Ramos C, Medeiros LJ, Wang SA |title=Precursor B-acute lymphoblastic leukemia occurring in patients with a history of prior malignancies: is it therapy-related? |journal=Haematologica |volume=97 |issue=6 |pages=919–25 |date=June 2012 |pmid=22207681 |pmc=3366660 |doi=10.3324/haematol.2011.057752 |url=}}</ref> | |||
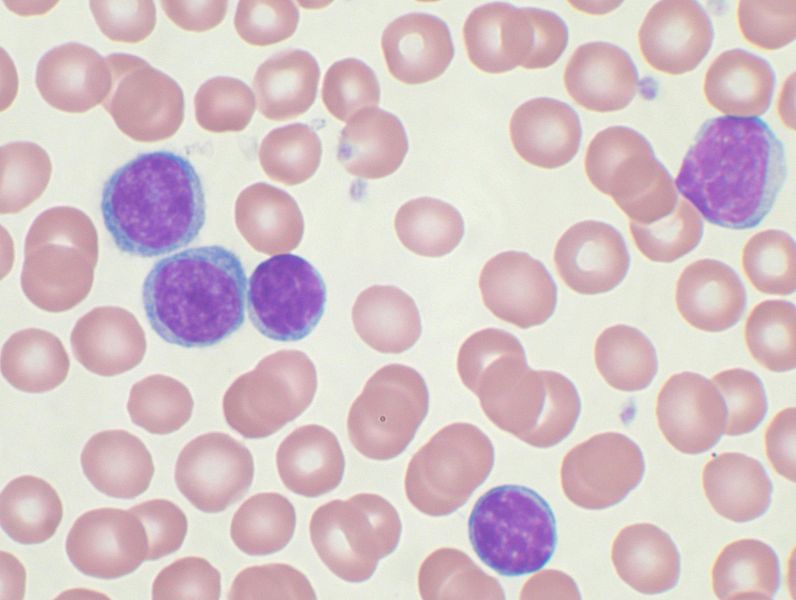
|'''[[Smudge cells]]''', condensed [[chromatin]], scant [[cytoplasm]], small [[nucleoli]]<ref name="pmid27221863">{{cite journal |vauthors=Chang CC, Sun JT, Liou TH, Kuo CF, Bei CH, Lin SJ, Tsai WT, Tan NC, Liou CB, Su MJ, Yen TH, Chu FY |title=Clinical Significance of Smudge Cells in Peripheral Blood Smears in Hematological Malignancies and Other Diseases |journal=Asian Pac. J. Cancer Prev. |volume=17 |issue=4 |pages=1847–50 |date=2016 |pmid=27221863 |doi= |url=}}</ref> | |||
|- | |||
|Cell involved | |||
|Immature B or [[T cells]] | |||
|Peripheral B or [[T cells]] | |||
|- | |||
|Clinical presentation | |||
|Stormy onset, symptoms related to depressed marrow function, bone pain, [[CNS]] manifestations. | |||
|Asymptomatic or nonspecific, [[hepatosplenomegaly]] , [[lymphadenopathy]] | |||
|- | |||
|Demographic | |||
|Most common [[leukemia]] in children | |||
|Most common [[leukemia]] in adults, twice as common in men. | |||
|- | |||
|CBC result | |||
|Anemia, [[thrombocytopenia]], variable [[WBC]]'s, and [[lymphoblast]] > 30% | |||
|[[Lymphocytosis]] > 5000/ul , low [[platelets]] in 20-30% | |||
|- | |||
|CD markers | |||
|[[CD19]], [[CD79a]], [[CD22]] (cytoplasmic), [[CD24]], [[CD10]], [[PAX5]], and TdT (terminal deoxyteransferase) | |||
|[[CD5]], CD19, [[CD20]] (dim), CD 23, and an absence of FMC-7 staining<ref name="pmid10403005">{{cite journal |vauthors=Tiensiwakul P, Lertlum T, Nuchprayoon I, Seksarn P |title=Immunophenotyping of acute lymphoblastic leukemia in pediatric patients by three-color flow cytometric analysis |journal=Asian Pac. J. Allergy Immunol. |volume=17 |issue=1 |pages=17–21 |date=March 1999 |pmid=10403005 |doi= |url=}}</ref> | |||
|} | |||
==References== | ==References== | ||
{{Reflist|2}} | {{Reflist|2}} | ||
Latest revision as of 15:18, 5 October 2019
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Nima Nasiri, M.D.[2]
For patient information, click here
| Lymphoid leukemia | |
| ICD-10 | C91 |
|---|---|
| ICD-9 | 204 |
| MeSH | D007945 |
|
Lymphoid leukemia Main Page |
Overview
Lymphoid leukemia is a monoclonal disorder which is a result of clonal proliferation and progressive accumulation of functionally incompetent lymphocytes in circulation, bone marrow, spleen, lymphoid tissues. Lymphocytic leukemia is more frequent than non-lymphocytic and other myeloproliferative diseases. The chronic form (CLL) affects older adult, onset is insidious. Acute lymphoblastic leukemia (ALL) is more common in children, peak incidence around 2 to 3 years of age. The identification of cytogenetic abnormalities is highly relevant for the prognosis of ALL. Patients may have findings associated with anemia, neutropenia, and/or thrombocytopenia due to bone marrow involvement. The white blood cell count may be decreased, normal, or markedly elevated. Symptoms can include fatigue, infections, or easy/spontaneous bruising or bleeding. Arthralgias and constitutional symptoms (eg, fever, night sweats, unintentional weight loss) are often present but are generally mild. Hepatomegaly, splenomegaly, and/or lymphadenopathy can be seen as well. Central nervous system (CNS) involvement may present as cranial neuropathies or meningeal symptoms. Lymphoblasts can have different surface molecules called cluster of differentiation (CD) which can be detected by flow cytometry.
Classification
ALL classification
There are two types of classifications for acute lymphoblastic leukemia : World Health Organization (WHO) and French-American-British (FAB)[1][2][3][4]
WHO classification of acute lymphoblastic leukemia
- B lymphoblastic leukemia/lymphoma:
- B lymphoblastic leukemia/lymphoma, Not otherwise specified
- B lymphoblastic leukemia/lymphoma with recurrent genetic abnormalities:
- B lymphoblastic leukemia/lymphoma with t(9;22),BCR-ABL[5]
- B lymphoblastic leukemia/lymphoma t(11q23); MLL rearrangement[6]
- B lymphoblastic leukemia/lymphoma with t(12;21)[7]
- B lymphoblastic leukemia/lymphoma with hyperdiploidy
- B lymphoblastic leukemia/lymphoma with hypodiploidy[8]
- B lymphoblastic leukemia/lymphoma t(5;14)
- B lymphoblastic leukemia/lymphoma t(1;19)
- T lymphoblastic leukemia/lymphoma:
FAB classification of acute lymphoblastic leukemia (for historical purposes):[9]
- ALL-L1: Small cells with homogeneous nuclear chromatin, a regular nuclear shape, small or no nucleoli, scanty cytoplasm, and mild to moderate basophilia
- ALL-L2: Large, heterogeneous cells with variable nuclear chromatin, an irregular nuclear shape, one or more nucleoli, a variable amount of cytoplasm, and basophilia
- ALL-L3: Large, homogeneous cells with fine, stippled chromatin; regular nuclei; prominent nucleoli; and abundant, deeply basophilic cytoplasm. The most distinguishing feature is prominent cytoplasmic vacuolation.
CLL classification
- There are two staging systems in order to classify CLL:
- Rai staging system (this is used more often in the United States, it is based on lymphocytosis)
- Stage Characteristics:
- Low Risk (Stage 0): Abnormal increase in the number of lymphocytes in the blood and marrow.
- Intermediate Risk (Stages I & II): Abnormal increase in the number of lymphocytes in the blood and the marrow, enlarged lymph nodes or abnormal increase in the number of lymphocytes in the circulating blood and the marrow, enlarged spleen and/or liver.
- High Risk (Stages III & IV): Abnormal increase in the number of lymphocytes in the circulating blood and the marrow, anemia (hemoglobin <11g/dL) or abnormal increase in the number of lymphocytes in the circulating blood and the marrow thrombocytopenia (platelets counts <100,000/uL).
- Binet staging system (this is more often used in Europe) [10]
- In the Binet staging system, CLL is classified by the number of affected lymphoid tissue groups (neck lymph nodes, groin lymph nodes, underarm lymph nodes, spleen, and liver) and by whether or not the patient has anemia (too few red blood cells) or thrombocytopenia (too few blood platelets).[11]
- Binet stage A: Fewer than 3 areas of lymphoid tissue are enlarged, with no anemia or thrombocytopenia.
- Binet stage B: 3 or more areas of lymphoid tissue are enlarged, with no anemia or thrombocytopenia.
- Binet stage C: Anemia and/or thrombocytopenia are present. Any number of lymphoid tissue areas may be enlarged.
- In the Binet staging system, CLL is classified by the number of affected lymphoid tissue groups (neck lymph nodes, groin lymph nodes, underarm lymph nodes, spleen, and liver) and by whether or not the patient has anemia (too few red blood cells) or thrombocytopenia (too few blood platelets).[11]
Pathophysiology
Physiology
- All lymphocytes have a common lymphoid progenitor cell origin known as a lymphoblast, the formation of lymphocytes is known as lymphopoiesis.
- B cells mature into B lymphocytes in the bone marrow, while T cells migrate to and mature in the thymus. Following maturation, the lymphocytes enter the circulation and peripheral lymphoid organs, where they survey for invading pathogens and cancer cells.
- The lymphocytes involved in adaptive immunity (B and T cells) differentiate further after exposure to an antigen, which occurs in the lymph nodes during antigen presentation from the dendritic cells.[12]
- The fully differentiated B and T cells are specific to the presented antigen and work to defend the body against pathogens associated with that antigen.[13][14]
- Lymphoid tissues are subdivided into primary and secondary lymphoid organs:[15][16][17]
- The primary lymphoid tissues responsible for the initial generation of B and T lymphocytes are the bone marrow and thymus, respectively.
- Secondary lymphoid tissues include lymph nodes, spleen, tonsils, gut-associated lymphoid tissue (GALT), bronchus-associated lymphoid tissue ( BALT).
- Within these lymphoid organs, B and T lymphocytes tend to home to different domains, leading to the segregation of B and T cells.
- Specifically, B cells mainly localize to follicles, whereas T cells mainly localize to interfollicular areas.
- Non-lymphoid cells (eg, dendritic cells, monocytes/macrophages, endothelial cells, and follicular dendritic cells) contribute to the formation of these distinct microenvironments, within which specific cell-cell interactions occur that are required for the generation of cellular and humoral immune responses.
Pathogenesis
- It is understood that lymphoid leukemia is a result of overproduction of cells which is caused by either activation or inactivation of genes.[18][19][20][21]
- Several factors, such as chromosomal translocations as well as genetic or epigenetic alterations, are involved in leukemogenesis.
- Abnormal methylation of DNA and histone modifications are important mechanisms in tumor suppressor silencing, contributing to leukemogenesis along with genetic alterations.[22]
- The activation of oncogenes involves genetic changes to cellular proto-oncogenes.
- Three genetic mechanisms activate oncogenes in human neoplasms, these mechanisms result in either an alteration of proto-oncogene structure or an increase in proto-oncogene expression:
- Mutation
- Gene amplification
- Chromosome rearrangements
Genetics
- Activation or/and inactivation of genes plays an important role in the pathogenesis and prognosis of lymphoid leukemia.
- Epigenetic and genetic alterations are two mechanisms in leukemia.
- Abnormal methylation of DNA and histone modifications are important mechanisms in tumor suppressor silencing, contributing to leukemogenesis along with genetic alterations.
- Epigenetic mechanisms are the most prevalent inactivation ones in lymphoid leukemia and involve the genes implicated in several cellular mechanisms, including gene expression and transcription, cell-cycle regulation and apoptosis.[23]
Associated Conditions
Conditions associated with lymphoid leukemia include:[24]
- Anemia
- Other cancers such as melanoma
- Lymph node enlargement
- Low grade fever
- Unexplained weight loss
- Night sweats
- Enlarged spleen or liver
- Infections of the skin, lungs, kidneys or other sites, as result of low immunoglobulin levels and decreased neutrophil counts.
- Fatigue
- Shortness of breath during normal physical activity
Differentiating Lymphoid Leukemia
| Characteristics | ALL | CLL |
|---|---|---|
| Microscopy |  |
 |
| Age of onset | Children (age < 10 years old) | Adult onset |
| Etiology | Chromosomal aberration resulting in abnormal transcription factors that affect development of B and T cells | Chromosomal deletion or possible somatic mutation of naive B cells |
| Morphology | Scanty, basophilic cytoplasm sometimes with a single long projection (‘hand-mirror cell’), condensed chromatin, small nucleoli[25][26] | Smudge cells, condensed chromatin, scant cytoplasm, small nucleoli[27] |
| Cell involved | Immature B or T cells | Peripheral B or T cells |
| Clinical presentation | Stormy onset, symptoms related to depressed marrow function, bone pain, CNS manifestations. | Asymptomatic or nonspecific, hepatosplenomegaly , lymphadenopathy |
| Demographic | Most common leukemia in children | Most common leukemia in adults, twice as common in men. |
| CBC result | Anemia, thrombocytopenia, variable WBC's, and lymphoblast > 30% | Lymphocytosis > 5000/ul , low platelets in 20-30% |
| CD markers | CD19, CD79a, CD22 (cytoplasmic), CD24, CD10, PAX5, and TdT (terminal deoxyteransferase) | CD5, CD19, CD20 (dim), CD 23, and an absence of FMC-7 staining[28] |
References
- ↑ Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz AD, Jaffe ES (May 2016). "The 2016 revision of the World Health Organization classification of lymphoid neoplasms". Blood. 127 (20): 2375–90. doi:10.1182/blood-2016-01-643569. PMC 4874220. PMID 26980727.
- ↑ Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J, Lister TA, Bloomfield CD (December 1999). "The World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues. Report of the Clinical Advisory Committee meeting, Airlie House, Virginia, November, 1997". Ann. Oncol. 10 (12): 1419–32. PMID 10643532.
- ↑ Makar AB, McMartin KE, Palese M, Tephly TR, Schmoldt A, Benthe HF, Haberland G, Anke H, Spector LB (June 1975). "Formate assay in body fluids: application in methanol poisoning". Biochem Med. 13 (2): 117–26. PMC 5922622. PMID 1.
- ↑ Wang Y, Miller S, Roulston D, Bixby D, Shao L (July 2016). "Genome-Wide Single-Nucleotide Polymorphism Array Analysis Improves Prognostication of Acute Lymphoblastic Leukemia/Lymphoma". J Mol Diagn. 18 (4): 595–603. doi:10.1016/j.jmoldx.2016.03.004. PMID 27161658.
- ↑ Goud TM, Al Salmani KK, Al Harasi SM, Al Musalhi M, Wasifuddin SM, Rajab A (2015). "Importance of FISH combined with Morphology, Immunophenotype and Cytogenetic Analysis of Childhood/ Adult Acute Lymphoblastic Leukemia in Omani Patients". Asian Pac. J. Cancer Prev. 16 (16): 7343–50. PMID 26514535.
- ↑ Nagayama J, Tomizawa D, Koh K, Nagatoshi Y, Hotta N, Kishimoto T, Takahashi Y, Kuno T, Sugita K, Sato T, Kato K, Ogawa A, Nakahata T, Mizutani S, Horibe K, Ishii E (June 2006). "Infants with acute lymphoblastic leukemia and a germline MLL gene are highly curable with use of chemotherapy alone: results from the Japan Infant Leukemia Study Group". Blood. 107 (12): 4663–5. doi:10.1182/blood-2005-11-4728. PMID 16478880.
- ↑ Peter A, Heiden T, Taube T, Körner G, Seeger K (November 2009). "Interphase FISH on TEL/AML1 positive acute lymphoblastic leukemia relapses--analysis of clinical relevance of additional TEL and AML1 copy number changes". Eur. J. Haematol. 83 (5): 420–32. doi:10.1111/j.1600-0609.2009.01315.x. PMID 19594616.
- ↑ Greipp PR, Trendle MC, Leong T, Oken MM, Kay NE, Van Ness B, Kyle RA (September 1999). "Is flow cytometric DNA content hypodiploidy prognostic in multiple myeloma?". Leuk. Lymphoma. 35 (1–2): 83–9. doi:10.3109/10428199909145707. PMID 10512165.
- ↑ Canaani J, Beohou E, Labopin M, Socié G, Huynh A, Volin L, Cornelissen J, Milpied N, Gedde-Dahl T, Deconinck E, Fegueux N, Blaise D, Mohty M, Nagler A (April 2017). "Impact of FAB classification on predicting outcome in acute myeloid leukemia, not otherwise specified, patients undergoing allogeneic stem cell transplantation in CR1: An analysis of 1690 patients from the acute leukemia working party of EBMT". Am. J. Hematol. 92 (4): 344–350. doi:10.1002/ajh.24640. PMID 28052366.
- ↑ Li H, Yi SH, Xiong WJ, Liu HM, Lyu R, Wang TY, Liu W, Zhong SZ, Yu Z, Zou DH, Xu Y, An G, Li ZJ, Qiu LG (January 2017). "Chronic Lymphocytic Leukemia Prognostic Index: A New Integrated Scoring System to Predict the Time to First Treatment in Chinese Patients with Chronic Lymphocytic Leukemia". Chin. Med. J. 130 (2): 135–142. doi:10.4103/0366-6999.197978. PMC 5282668. PMID 28091403.
- ↑ Delgado J, Doubek M, Baumann T, Kotaskova J, Molica S, Mozas P, Rivas-Delgado A, Morabito F, Pospisilova S, Montserrat E (April 2017). "Chronic lymphocytic leukemia: A prognostic model comprising only two biomarkers (IGHV mutational status and FISH cytogenetics) separates patients with different outcome and simplifies the CLL-IPI". Am. J. Hematol. 92 (4): 375–380. doi:10.1002/ajh.24660. PMID 28120419.
- ↑ van de Loosdrecht AA, van Wetering S, Santegoets S, Singh SK, Eeltink CM, den Hartog Y, Koppes M, Kaspers J, Ossenkoppele GJ, Kruisbeek AM, de Gruijl TD (October 2018). "A novel allogeneic off-the-shelf dendritic cell vaccine for post-remission treatment of elderly patients with acute myeloid leukemia". Cancer Immunol. Immunother. 67 (10): 1505–1518. doi:10.1007/s00262-018-2198-9. PMC 6182404. PMID 30039426. Vancouver style error: initials (help)
- ↑ Khoury HJ, Collins RH, Blum W, Stiff PS, Elias L, Lebkowski JS, Reddy A, Nishimoto KP, Sen D, Wirth ED, Case CC, DiPersio JF (August 2017). "Immune responses and long-term disease recurrence status after telomerase-based dendritic cell immunotherapy in patients with acute myeloid leukemia". Cancer. 123 (16): 3061–3072. doi:10.1002/cncr.30696. PMID 28411378.
- ↑ Cesta MF (2006). "Normal structure, function, and histology of mucosa-associated lymphoid tissue". Toxicol Pathol. 34 (5): 599–608. doi:10.1080/01926230600865531. PMID 17067945.
- ↑ Bertrand JY, Chi NC, Santoso B, Teng S, Stainier DY, Traver D (March 2010). "Haematopoietic stem cells derive directly from aortic endothelium during development". Nature. 464 (7285): 108–11. doi:10.1038/nature08738. PMC 2858358. PMID 20154733.
- ↑ Dorshkind K, Montecino-Rodriguez E (March 2007). "Fetal B-cell lymphopoiesis and the emergence of B-1-cell potential". Nat. Rev. Immunol. 7 (3): 213–9. doi:10.1038/nri2019. PMID 17318232.
- ↑ Vossenkämper A, Blair PA, Safinia N, Fraser LD, Das L, Sanders TJ, Stagg AJ, Sanderson JD, Taylor K, Chang F, Choong LM, D'Cruz DP, Macdonald TT, Lombardi G, Spencer J (August 2013). "A role for gut-associated lymphoid tissue in shaping the human B cell repertoire". J. Exp. Med. 210 (9): 1665–74. doi:10.1084/jem.20122465. PMC 3754866. PMID 23940259.
- ↑ Quijada-Álamo M, Hernández-Sánchez M, Robledo C, Hernández-Sánchez JM, Benito R, Montaño A, Rodríguez-Vicente AE, Quwaider D, Martín AÁ, García-Álvarez M, Vidal-Manceñido MJ, Ferrer-Garrido G, Delgado-Beltrán MP, Galende J, Rodríguez JN, Martín-Núñez G, Alonso JM, García de Coca A, Queizán JA, Sierra M, Aguilar C, Kohlmann A, Hernández JÁ, González M, Hernández-Rivas JM (April 2017). "Next-generation sequencing and FISH studies reveal the appearance of gene mutations and chromosomal abnormalities in hematopoietic progenitors in chronic lymphocytic leukemia". J Hematol Oncol. 10 (1): 83. doi:10.1186/s13045-017-0450-y. PMC 5387353. PMID 28399885.
- ↑ Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J, Lister TA, Bloomfield CD (December 1999). "World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting-Airlie House, Virginia, November 1997". J. Clin. Oncol. 17 (12): 3835–49. doi:10.1200/JCO.1999.17.12.3835. PMID 10577857.
- ↑ Zhang S, Kipps TJ (2014). "The pathogenesis of chronic lymphocytic leukemia". Annu Rev Pathol. 9: 103–18. doi:10.1146/annurev-pathol-020712-163955. PMID 23987584.
- ↑ Ge H, Wu X, Shen J, Chen J, Chen Y, Zhang Y (July 2018). "A case report of extranodal NK/T-cell lymphoma in patient with chronic lymphocytic leukemia". Medicine (Baltimore). 97 (30): e11619. doi:10.1097/MD.0000000000011619. PMC 6078727. PMID 30045301.
- ↑ Kondo Y (August 2009). "Epigenetic cross-talk between DNA methylation and histone modifications in human cancers". Yonsei Med. J. 50 (4): 455–63. doi:10.3349/ymj.2009.50.4.455. PMC 2730606. PMID 19718392.
- ↑ Gladkikh AA, Potashnikova DM, Tatarskiy V, Yastrebova M, Khamidullina A, Barteneva N, Vorobjev I (December 2017). "Comparison of the mRNA expression profile of B-cell receptor components in normal CD5-high B-lymphocytes and chronic lymphocytic leukemia: a key role of ZAP70". Cancer Med. 6 (12): 2984–2997. doi:10.1002/cam4.1257. PMC 5727315. PMID 29125235.
- ↑ Ito Y, Makita S, Maeshima AM, Hatta S, Suzuki T, Yuda S, Fukuhara S, Munakata W, Suzuki T, Maruyama D, Izutsu K (August 2018). "Paraneoplastic Pemphigus Associated with B-cell Chronic Lymphocytic Leukemia Treated with Ibrutinib and Rituximab". Intern. Med. 57 (16): 2395–2398. doi:10.2169/internalmedicine.0578-17. PMC 6148183. PMID 29526963.
- ↑ Mazur EM, Wittels EG, Schiffman FJ, South K, Horner RJ (January 1986). "Hand mirror cell lymphoid leukemia in adults. A distinct clinicopathologic syndrome. Case report and literature review". Cancer. 57 (1): 92–9. PMID 3455677.
- ↑ Tang G, Zuo Z, Thomas DA, Lin P, Liu D, Hu Y, Kantarjian HM, Bueso-Ramos C, Medeiros LJ, Wang SA (June 2012). "Precursor B-acute lymphoblastic leukemia occurring in patients with a history of prior malignancies: is it therapy-related?". Haematologica. 97 (6): 919–25. doi:10.3324/haematol.2011.057752. PMC 3366660. PMID 22207681.
- ↑ Chang CC, Sun JT, Liou TH, Kuo CF, Bei CH, Lin SJ, Tsai WT, Tan NC, Liou CB, Su MJ, Yen TH, Chu FY (2016). "Clinical Significance of Smudge Cells in Peripheral Blood Smears in Hematological Malignancies and Other Diseases". Asian Pac. J. Cancer Prev. 17 (4): 1847–50. PMID 27221863.
- ↑ Tiensiwakul P, Lertlum T, Nuchprayoon I, Seksarn P (March 1999). "Immunophenotyping of acute lymphoblastic leukemia in pediatric patients by three-color flow cytometric analysis". Asian Pac. J. Allergy Immunol. 17 (1): 17–21. PMID 10403005.