Fibromuscular dysplasia pathophysiology: Difference between revisions
| (One intermediate revision by the same user not shown) | |||
| Line 134: | Line 134: | ||
==Microscopic Pathology== | ==Microscopic Pathology== | ||
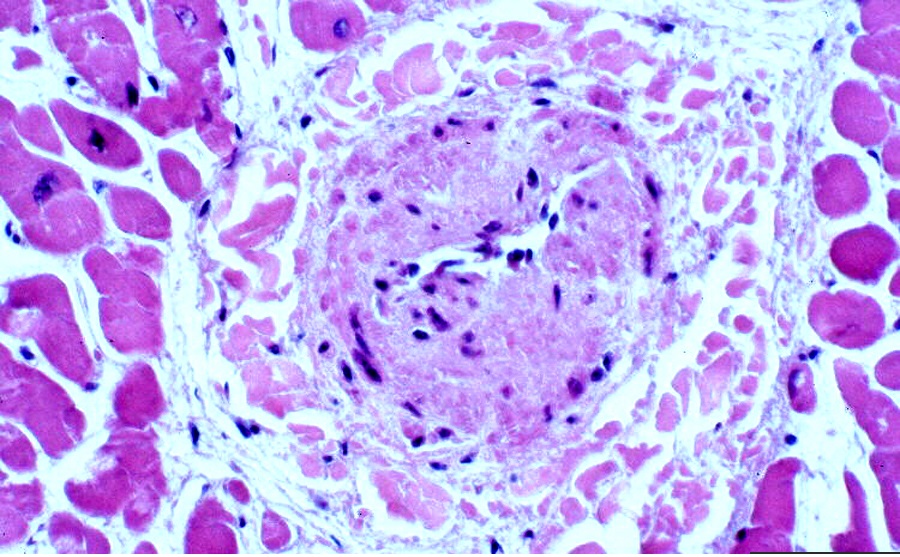
*On microscopic histopathological analysis, circumferential or eccentric deposition of collagen in intima but no lipid or inflammatory component are characteristic findings of FMD. | *On microscopic histopathological analysis, circumferential or eccentric deposition of collagen in intima but no lipid or inflammatory component are characteristic findings of FMD. | ||
[[File:FMD..jpg| | [[File:FMD..jpg||none|600px|thumb|Microscopic pathology Source:Librepathology]] | ||
==References== | ==References== | ||
Latest revision as of 18:56, 30 August 2018
|
Fibromuscular dysplasia Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
ASA/ACCF/AHA Guideline Recommendations |
|
Management of Patients With Fibromuscular Dysplasia of the Extracranial Carotid Arteries |
|
Case Studies |
|
Fibromuscular dysplasia pathophysiology On the Web |
|
American Roentgen Ray Society Images of Fibromuscular dysplasia pathophysiology |
|
Risk calculators and risk factors for Fibromuscular dysplasia pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Mohsen Basiri M.D.
Overview
In Fibromuscular dysplasia, the proliferation of vascular smooth muscle of one or more small or medium-sized arteries undergo dysplasia and cause stenosis. this abnormal cellular development is characterized by fibrous thickening of the intima, media, or adventitia of the involved arteries; which ultimately lead to arterial narrowing. Despite numerous genetic, hormonal and mechanical factors have been proposed, the etiology of fibromuscular dysplasia remains unknown.
Pathophysiology
In Fibromuscular dysplasia, the proliferation of vascular smooth muscle of one or more small or medium-sized arteries undergo dysplasia and cause stenosis. this abnormal cellular development is characterized by fibrous thickening of the intima, media, or adventitia of the involved arteries; which ultimately lead to arterial narrowing.
Pathogenesis
Despite numerous genetic, hormonal and mechanical factors have been proposed, the etiology of fibromuscular dysplasia remains unknown. A variety of factors have been implicated. These include:
- Cigarette smoking and a history of hypertension[1]
- Genetic factors, with a reported autosomal mode of inheritance in some families.[2]
- Some studies suggest fibromuscular dysplasia is a systemic disease with altered TGF-β expression and connective tissue features.
- Hormonal influence, The increased incidence of FMD in women as compared with men suggests a possible hormonal given the predominance in women of childbearing age but no association has been found between fibromuscular dysplasia and previous use of oral contraceptives or abnormalities of endogenous sex hormones.[3]
- Some authors have proposed the sex difference to be related to immune system functioning, but overt inflammation, as is observed in most classic autoimmune diseases, is histologically lacking.
- Mechanical factors due to stretching of smooth muscle cells and microtrauma to the vessel wall.
- Ischemia due to fibrotic occlusion of the vasa vasorum.
Genetics
- Genetic predisposition may play a role in the development of fibromuscular dysplasia. Owing to, FMD is more common among the first-degree relatives of patients with this condition.
- Some studies showed an autosomal dominant transmission pattern for fibromuscular dysplasia; however, as of yet, no etiologic genes have been identified for this disease. Applying molecular genetics investigations will reveal information about FMD pathogenesis, and family-based studies, evaluating genome of candidates, and wide genome studies can help to recognize pathophysiology of FMD.
- In the US Registry, about eight percent of patients report a confirmed diagnosis of FMD in one or more in first- or second-degree family members. However, the high prevalence of aneurysms, sudden death, and stroke among first- and second-degree family members in the US Registry shows that FMD may be associated with systemic arteriopathy with a great diversity of clinical phenotype traits. It is hypothesized that FMD may have common features with vascular connective tissue diseases, such as Loeys-Dietz syndrome or the vascular type of Ehlers-Danlos syndrome.
- Increased level of TGF-β1 and 2 secreted by fibroblasts in patients with FMD in comparison to matched
FMD patients also had elevated plasma levels of circulating TGF-β1 and TGF-β2 relative to matched controls. The potential involvement of TGF-b pathways in the pathogenesis of FMD is an area for future investigation, especially as this pathway could provide a potential target for disease-modifying medical therapies.[4]
- Polymorphisms of angiotensin-converting–enzyme allele ACE-I among patients with multifocal renal arterial fibromuscular
dysplasia has been investigated.[5]
- In some case reports, the association of FMD with neurofibromatosis, Alport syndrome, and pheochromocytoma have been considered; And mutations in collagen, and with alpha1-antitrypsin deficiency have also been suggested.[6][7][8]
Associated Conditions
- The underlying connective tissue problem among arteries with FMD, causes weakening of arterial wall and leads to vessel dilatation.The combined prevalence of aneurysms and FMD is estimated to be about 8%.
- FMD is a predisposing factor for spontaneous cervical carotid, or renal artery dissections. Dissection through the weakening of the arterial wall, in FMD are more commonly multiple than in patients without an identified underlying arteriopathy.
Gross Pathology
- On gross pathology, focal, irregular, thickening in medium and large muscular arteries are characteristic findings of FMD.
Microscopic Pathology
- On microscopic histopathological analysis, circumferential or eccentric deposition of collagen in intima but no lipid or inflammatory component are characteristic findings of FMD.
References
- ↑ C. N. Sang, P. K. Whelton, U. M. Hamper, M. Connolly, S. Kadir, R. I. White, R. Sanders, K. Y. Liang & W. Bias (1989). "Etiologic factors in renovascular fibromuscular dysplasia. A case-control study". Hypertension (Dallas, Tex. : 1979). 14 (5): 472–479. PMID 2680961. Unknown parameter
|month=ignored (help) - ↑ J. Perdu, P. Boutouyrie, C. Bourgain, N. Stern, B. Laloux, E. Bozec, M. Azizi, C. Bonaiti-Pellie, P.-F. Plouin, S. Laurent, A.-P. Gimenez-Roqueplo & X. Jeunemaitre (2007). "Inheritance of arterial lesions in renal fibromuscular dysplasia". Journal of human hypertension. 21 (5): 393–400. doi:10.1038/sj.jhh.1002156. PMID 17330059. Unknown parameter
|month=ignored (help) - ↑ C. N. Sang, P. K. Whelton, U. M. Hamper, M. Connolly, S. Kadir, R. I. White, R. Sanders, K. Y. Liang & W. Bias (1989). "Etiologic factors in renovascular fibromuscular dysplasia. A case-control study". Hypertension (Dallas, Tex. : 1979). 14 (5): 472–479. PMID 2680961. Unknown parameter
|month=ignored (help) - ↑ Santhi K. Ganesh, Rachel Morissette, Zhi Xu, Florian Schoenhoff, Benjamin F. Griswold, Jiandong Yang, Lan Tong, Min-Lee Yang, Kristina Hunker, Leslie Sloper, Shinie Kuo, Rafi Raza, Dianna M. Milewicz, Clair A. Francomano, Harry C. Dietz, Jennifer Van Eyk & Nazli B. McDonnell (2014). "Clinical and biochemical profiles suggest fibromuscular dysplasia is a systemic disease with altered TGF-beta expression and connective tissue features". FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 28 (8): 3313–3324. doi:10.1096/fj.14-251207. PMID 24732132. Unknown parameter
|month=ignored (help) - ↑ A. Bofinger, C. Hawley, P. Fisher, N. Daunt, M. Stowasser & R. Gordon (2001). "Polymorphisms of the renin-angiotensin system in patients with multifocal renal arterial fibromuscular dysplasia". Journal of human hypertension. 15 (3): 185–190. doi:10.1038/sj.jhh.1001144. PMID 11317203. Unknown parameter
|month=ignored (help) - ↑ David P. Slovut & Jeffrey W. Olin (2004). "Fibromuscular dysplasia". The New England journal of medicine. 350 (18): 1862–1871. doi:10.1056/NEJMra032393. PMID 15115832. Unknown parameter
|month=ignored (help) - ↑ G. Tromp, Y. Wu, D. J. Prockop, S. L. Madhatheri, C. Kleinert, J. J. Earley, J. Zhuang, O. Norrgard, R. C. Darling & W. M. Abbott (1993). "Sequencing of cDNA from 50 unrelated patients reveals that mutations in the triple-helical domain of type III procollagen are an infrequent cause of aortic aneurysms". The Journal of clinical investigation. 91 (6): 2539–2545. doi:10.1172/JCI116490. PMID 8514866. Unknown parameter
|month=ignored (help) - ↑ W. I. Schievink, F. B. Meyer, J. E. Parisi & E. F. Wijdicks (1998). "Fibromuscular dysplasia of the internal carotid artery associated with alpha1-antitrypsin deficiency". Neurosurgery. 43 (2): 229–233. PMID 9696074. Unknown parameter
|month=ignored (help)