11β-hydroxylase deficiency pathophysiology: Difference between revisions
| (26 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
__NOTOC__ | __NOTOC__ | ||
{{ | {{11β-hydroxylase deficiency}} | ||
{{CMG}}; {{AE}} {{ | |||
{{CMG}}; {{AE}} {{MJ}} | |||
==Overview== | ==Overview== | ||
11β-Hydroxylase deficiency is a type of [[congenital adrenal hyperplasia]] resulting from a defect in [[CYP11B1]] on [[chromosome 8]]. [[CYP11B1]] [[gene]] encodes an enzyme called [[11β-hydroxylase]] in the path of [[steroid biosynthesis]]. This enzyme is located in the zona fasciculate, and converts [[Deoxycortisol|11-deoxycortisol]] to [[cortisol]] and [[11-deoxycorticosterone]]. Lack of [[11β-hydroxylase]] enzyme in different amounts results in accumulation of [[Deoxycortisol|11-deoxycortisol]], and decrease amounts of [[cortisol]] and [[11-deoxycorticosterone]]. There is an elevation of [[adrenocorticotropic hormone]] results in overproduction of [[11-deoxycorticosterone]] (DOC) by mid-childhood. [[11-deoxycorticosterone]] is a weak [[mineralocorticoid]], but because of high amounts in this disease can cause [[mineralocorticoid excess]] effects such as salt retention, volume expansion, and [[hypertension]]. Non-classic forms mostly doesn't have verifiable [[mutations]] and mild 11β-hydroxylase deficiency is currently considered a very rare cause of [[hirsutism]] and [[infertility]]. | |||
==Pathogenesis== | ==Pathogenesis== | ||
* 11β- | * [[11β-hydroxylase]] deficiency is a type of [[congenital adrenal hyperplasia]] resulting from a defect in [[CYP11B1]] on [[chromosome 8]]. | ||
* [[CYP11B1]] [[gene]] encodes an enzyme called [[11β-hydroxylase]] in the path of [[steroid biosynthesis]]. This [[enzyme]] is located in the [[zona fasciculata]], and converts [[Deoxycortisol|11-deoxycortisol]] to [[cortisol]] and [[11-deoxycorticosterone]]. | |||
* [[ | * [[Cortisol]] production reduction has a negative feedback on the [[pituitary]] and increases [[corticotropin]] ([[ACTH]]) secretion. This leads to of [[Deoxycortisol|11-deoxycortisol]] [[precursors]] and then [[adrenocortical hyperplasia]]. | ||
* | * With intact amount of other pathways, as a result of high [[ACTH]] concentrations, some amount of the [[Deoxycortisol|11-deoxycortisol]] precursors are metabolized to [[adrenal]] [[androgens]] and can cause [[virilization]] in a genetically female fetus or a child of either sex. | ||
* | * Severity of disease depends on the amount of functional [[11β-hydroxylase]] [[enzyme]] that an individual produces. | ||
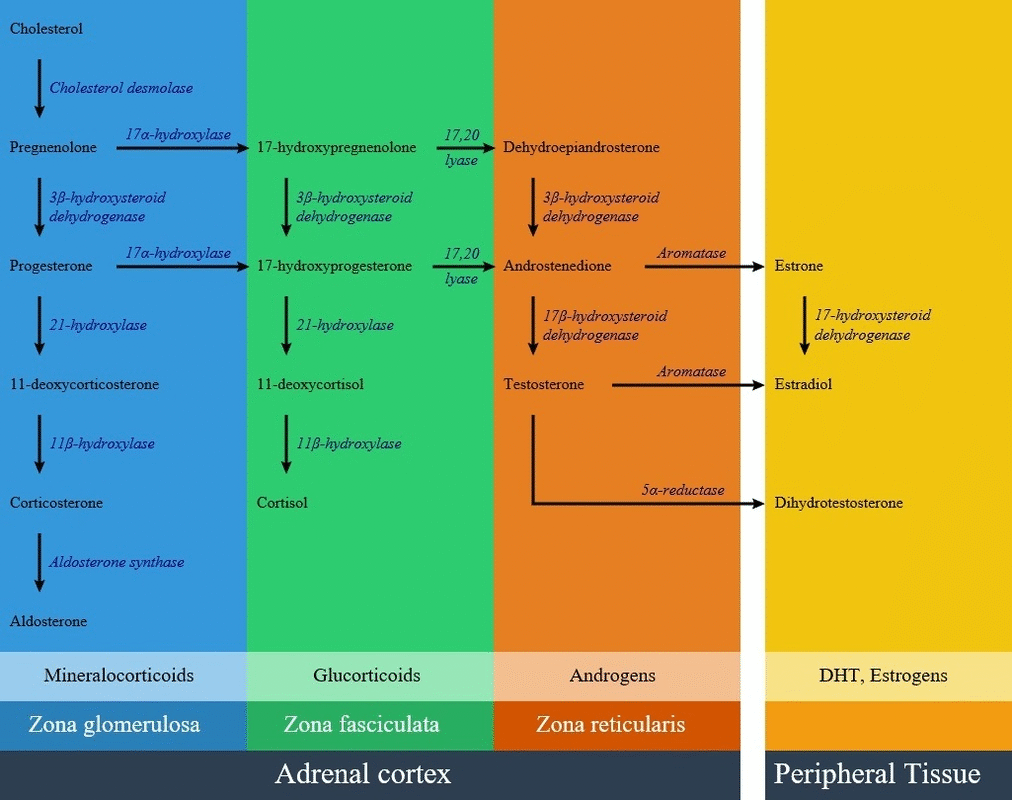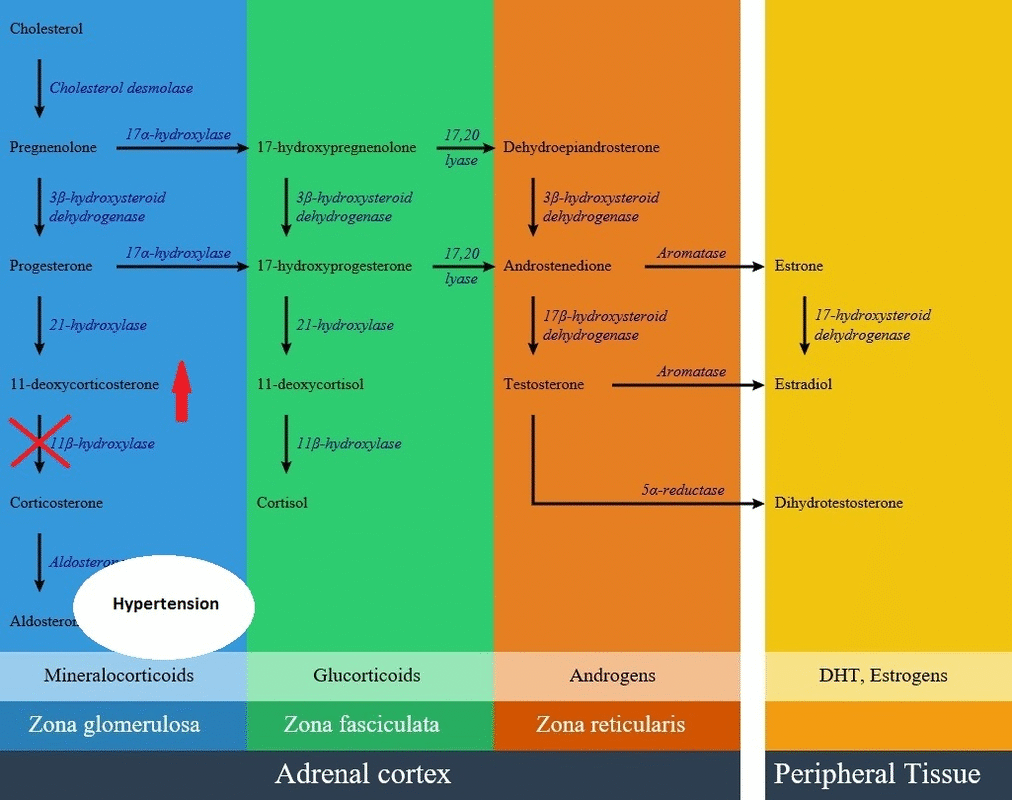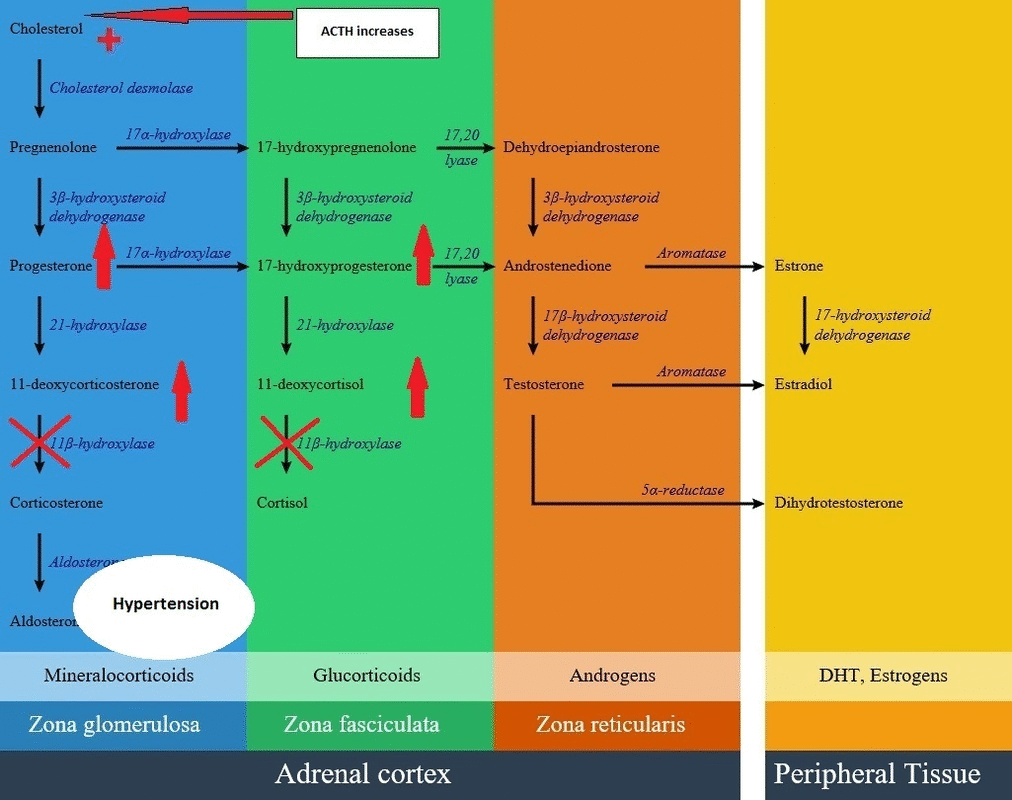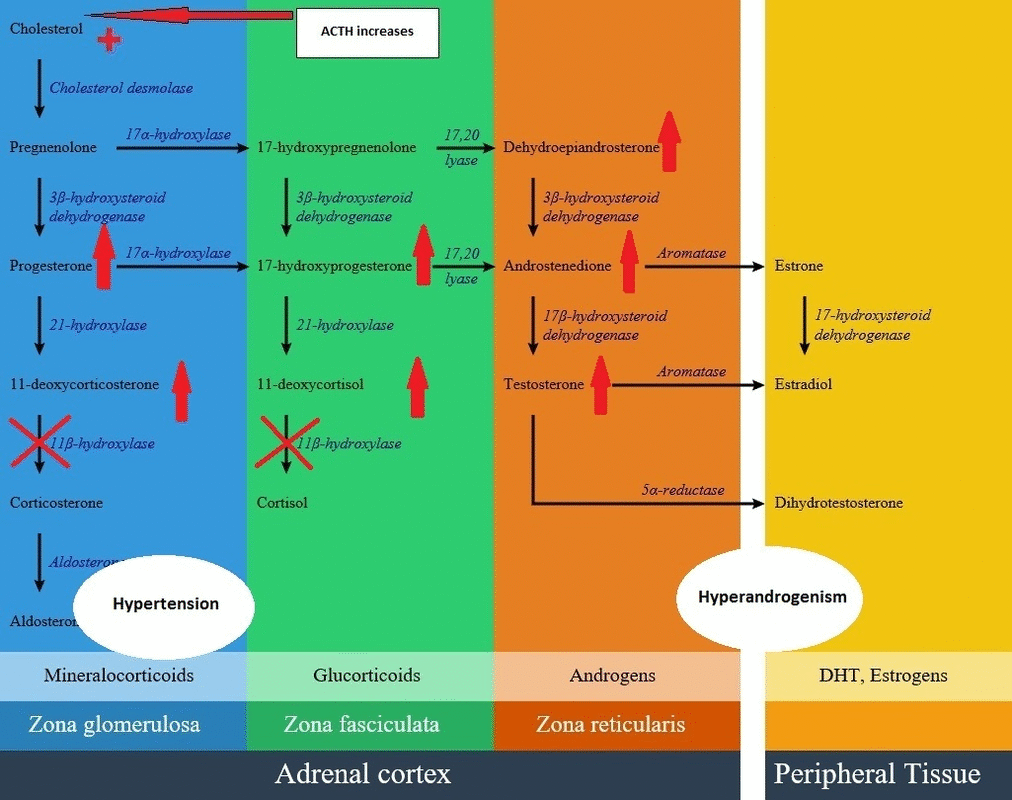
* [[Aldosterone]] production is decreased in this disease but there is an elevation of [[adrenocorticotropic hormone]] results in overproduction of [[11-deoxycorticosterone]] (DOC) by mid-childhood. [[11-deoxycorticosterone]] is a weak [[mineralocorticoid]], but because of high amounts in this disease can cause [[mineralocorticoid excess]] effects such as [[salt]] retention, volume expansion, and [[hypertension]]. | |||
* Non-classic forms mostly doesn't have verifiable [[mutations]] and mild [[11β-hydroxylase]] deficiency is currently considered a very rare cause of [[hirsutism]] and [[infertility]]. | |||
[[image:11- hydroxylase.gif|center|frame|800px|Adrenal steroid synthesis pathways in adrenal cortex and related enzymes <ref name="urlFile:Adrenal Steroids Pathways.svg - Wikimedia Commons">{{cite web |url=https://commons.wikimedia.org/wiki/File:Adrenal_Steroids_Pathways.svg|title=File:Adrenal Steroids Pathways.svg - Wikimedia Commons |format= |work= |accessdate=}}</ref>]] | |||
==Genetics== | ==Genetics== | ||
* | * [[11β-hydroxylase]] deficiency is an [[inherited]] [[disease]] with an [[autosomal recessive]] pattern, which means both copies of the [[gene]] in each cell have [[gene]] [[mutations]]. | ||
* | * Commonly, the parents of an individual with an [[autosomal recessive]] condition each carry one copy of the mutated [[gene]], but they typically do not show signs and symptoms of the condition. | ||
* Most [[CYP11B1]] [[mutations]] correspond to minimal or absent [[enzyme activity]], resulting in the classic 11β-hydroxylase deficiency phenotype. | |||
* A non-classic form of enzyme deficiency caused by [[CYP11B1]] mutations exists but is very rare.<ref name="pmid28576284">{{cite journal |vauthors=El-Maouche D, Arlt W, Merke DP |title=Congenital adrenal hyperplasia |journal=Lancet |volume= |issue= |pages= |year=2017 |pmid=28576284 |doi=10.1016/S0140-6736(17)31431-9 |url=}}</ref><ref name="pmid6296182">{{cite journal |vauthors=Zachmann M, Tassinari D, Prader A |title=Clinical and biochemical variability of congenital adrenal hyperplasia due to 11 beta-hydroxylase deficiency. A study of 25 patients |journal=J. Clin. Endocrinol. Metab. |volume=56 |issue=2 |pages=222–9 |year=1983 |pmid=6296182 |doi=10.1210/jcem-56-2-222 |url=}}</ref><ref name="pmid28476231">{{cite journal |vauthors=Hannah-Shmouni F, Chen W, Merke DP |title=Genetics of Congenital Adrenal Hyperplasia |journal=Endocrinol. Metab. Clin. North Am. |volume=46 |issue=2 |pages=435–458 |year=2017 |pmid=28476231 |doi=10.1016/j.ecl.2017.01.008 |url=}}</ref> | |||
==Associated Conditions== | ==Associated Conditions== | ||
* Hypertension | * [[Hirsutism]] | ||
* Testicular tumor | * [[Hypertension]] | ||
* [[Testicular tumor]] | |||
==Gross Pathology== | ==Gross Pathology== | ||
[[Gross pathology]] findings in patients with 11β-hydroxylase deficiency are:<ref name="radio">Congenital adrenal hyperplasia. Dr Henry Knipe and Dr M Venkatesh . Radiopaedia.org 2015.http://radiopaedia.org/articles/congenital-adrenal-hyperplasia</ref><ref name="pmid25372578">{{cite journal |vauthors=Teixeira SR, Elias PC, Andrade MT, Melo AF, Elias Junior J |title=The role of imaging in congenital adrenal hyperplasia |journal=Arq Bras Endocrinol Metabol |volume=58 |issue=7 |pages=701–8 |year=2014 |pmid=25372578 |doi= |url=}}</ref> | |||
*Enlarged [[adrenal glands]] | |||
*Wrinkled surface [[adrenal glands]] | |||
*Cerebriform pattern [[adrenal glands]] ([[pathognomonic]] sign) | |||
*Normal [[ultrasound]] appearances may also be seen | |||
*[[Testicular]] masses may be identified representing adrenal rest tissue | |||
[[Image:Cah.jpg|center|thumb|400px|frame|Adrenal gland, Cortex - Hyperplasia in a male rat from a chronic study. There are two adjacent foci of hyperplasia (H) in the zona fasciculata.<ref name="urlAdrenal Gland - Hyperplasia - Nonneoplastic Lesion Atlas">{{cite web |url=https://ntp.niehs.nih.gov/nnl/endocrine/adrenal/hyperpl/index.htm |title=Adrenal Gland - Hyperplasia - Nonneoplastic Lesion Atlas |format= |work= |accessdate=}}</ref>]] | |||
==Microscopic Pathology== | ==Microscopic Pathology== | ||
* Diffuse cortical hyperplasia | In 11β-hydroxylase deficiency [[microscopic]] findings may include: | ||
* | * Diffuse cortical [[hyperplasia]] with smaller [[cells]] | ||
* The cell [[cytoplasm]] can be vacuolated, and often more [[basophilic]] | |||
* Rare [[mitotic]] figures may be present | |||
* The [[hyperplastic]] cells typically lack features of cellular [[atypia]].<ref name="urlAdrenal Gland - Hyperplasia - Nonneoplastic Lesion Atlas">{{cite web |url=https://ntp.niehs.nih.gov/nnl/endocrine/adrenal/hyperpl/index.htm |title=Adrenal Gland - Hyperplasia - Nonneoplastic Lesion Atlas |format= |work= |accessdate=}}</ref> | |||
[[Image:Cah mic.jpg|center|thumb|400px|frame|Adrenal gland, Cortex - Hyperplasia in a female rat from a chronic study. There is a hyperplastic lesion (H) in which cortical cells are increased in number but are smaller in size than adjacent normal cortical cells (NC)<ref name="urlAdrenal Gland - Hyperplasia - Nonneoplastic Lesion Atlas">{{cite web |url=https://ntp.niehs.nih.gov/nnl/endocrine/adrenal/hyperpl/index.htm |title=Adrenal Gland - Hyperplasia - Nonneoplastic Lesion Atlas |format= |work= |accessdate=}}</ref>]] | |||
==References== | ==References== | ||
{{Reflist|2}} | {{Reflist|2}} | ||
Latest revision as of 19:36, 18 October 2017
|
11β-hydroxylase deficiency Microchapters |
|
Differentiating 11β-hydroxylase deficiency from other Diseases |
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
11β-hydroxylase deficiency pathophysiology On the Web |
|
American Roentgen Ray Society Images of 11β-hydroxylase deficiency pathophysiology |
|
Directions to Hospitals Treating Congenital adrenal hyperplasia due to 11β-hydroxylase deficiency |
|
Risk calculators and risk factors for 11β-hydroxylase deficiency pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Mehrian Jafarizade, M.D [2]
Overview
11β-Hydroxylase deficiency is a type of congenital adrenal hyperplasia resulting from a defect in CYP11B1 on chromosome 8. CYP11B1 gene encodes an enzyme called 11β-hydroxylase in the path of steroid biosynthesis. This enzyme is located in the zona fasciculate, and converts 11-deoxycortisol to cortisol and 11-deoxycorticosterone. Lack of 11β-hydroxylase enzyme in different amounts results in accumulation of 11-deoxycortisol, and decrease amounts of cortisol and 11-deoxycorticosterone. There is an elevation of adrenocorticotropic hormone results in overproduction of 11-deoxycorticosterone (DOC) by mid-childhood. 11-deoxycorticosterone is a weak mineralocorticoid, but because of high amounts in this disease can cause mineralocorticoid excess effects such as salt retention, volume expansion, and hypertension. Non-classic forms mostly doesn't have verifiable mutations and mild 11β-hydroxylase deficiency is currently considered a very rare cause of hirsutism and infertility.
Pathogenesis
- 11β-hydroxylase deficiency is a type of congenital adrenal hyperplasia resulting from a defect in CYP11B1 on chromosome 8.
- CYP11B1 gene encodes an enzyme called 11β-hydroxylase in the path of steroid biosynthesis. This enzyme is located in the zona fasciculata, and converts 11-deoxycortisol to cortisol and 11-deoxycorticosterone.
- Cortisol production reduction has a negative feedback on the pituitary and increases corticotropin (ACTH) secretion. This leads to of 11-deoxycortisol precursors and then adrenocortical hyperplasia.
- With intact amount of other pathways, as a result of high ACTH concentrations, some amount of the 11-deoxycortisol precursors are metabolized to adrenal androgens and can cause virilization in a genetically female fetus or a child of either sex.
- Severity of disease depends on the amount of functional 11β-hydroxylase enzyme that an individual produces.
- Aldosterone production is decreased in this disease but there is an elevation of adrenocorticotropic hormone results in overproduction of 11-deoxycorticosterone (DOC) by mid-childhood. 11-deoxycorticosterone is a weak mineralocorticoid, but because of high amounts in this disease can cause mineralocorticoid excess effects such as salt retention, volume expansion, and hypertension.
- Non-classic forms mostly doesn't have verifiable mutations and mild 11β-hydroxylase deficiency is currently considered a very rare cause of hirsutism and infertility.
Genetics
- 11β-hydroxylase deficiency is an inherited disease with an autosomal recessive pattern, which means both copies of the gene in each cell have gene mutations.
- Commonly, the parents of an individual with an autosomal recessive condition each carry one copy of the mutated gene, but they typically do not show signs and symptoms of the condition.
- Most CYP11B1 mutations correspond to minimal or absent enzyme activity, resulting in the classic 11β-hydroxylase deficiency phenotype.
- A non-classic form of enzyme deficiency caused by CYP11B1 mutations exists but is very rare.[2][3][4]
Associated Conditions
Gross Pathology
Gross pathology findings in patients with 11β-hydroxylase deficiency are:[5][6]
- Enlarged adrenal glands
- Wrinkled surface adrenal glands
- Cerebriform pattern adrenal glands (pathognomonic sign)
- Normal ultrasound appearances may also be seen
- Testicular masses may be identified representing adrenal rest tissue

Microscopic Pathology
In 11β-hydroxylase deficiency microscopic findings may include:
- Diffuse cortical hyperplasia with smaller cells
- The cell cytoplasm can be vacuolated, and often more basophilic
- Rare mitotic figures may be present
- The hyperplastic cells typically lack features of cellular atypia.[7]

References
- ↑ "File:Adrenal Steroids Pathways.svg - Wikimedia Commons".
- ↑ El-Maouche D, Arlt W, Merke DP (2017). "Congenital adrenal hyperplasia". Lancet. doi:10.1016/S0140-6736(17)31431-9. PMID 28576284.
- ↑ Zachmann M, Tassinari D, Prader A (1983). "Clinical and biochemical variability of congenital adrenal hyperplasia due to 11 beta-hydroxylase deficiency. A study of 25 patients". J. Clin. Endocrinol. Metab. 56 (2): 222–9. doi:10.1210/jcem-56-2-222. PMID 6296182.
- ↑ Hannah-Shmouni F, Chen W, Merke DP (2017). "Genetics of Congenital Adrenal Hyperplasia". Endocrinol. Metab. Clin. North Am. 46 (2): 435–458. doi:10.1016/j.ecl.2017.01.008. PMID 28476231.
- ↑ Congenital adrenal hyperplasia. Dr Henry Knipe and Dr M Venkatesh . Radiopaedia.org 2015.http://radiopaedia.org/articles/congenital-adrenal-hyperplasia
- ↑ Teixeira SR, Elias PC, Andrade MT, Melo AF, Elias Junior J (2014). "The role of imaging in congenital adrenal hyperplasia". Arq Bras Endocrinol Metabol. 58 (7): 701–8. PMID 25372578.
- ↑ 7.0 7.1 7.2 "Adrenal Gland - Hyperplasia - Nonneoplastic Lesion Atlas".