Mebendazole
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Mebendazole is a broad-spectrum anthelmintic that is FDA approved for the treatment of Enterobius vermicularis (pinworm), Trichuris trichiura (whipworm), Ascaris lumbricoides (common roundworm), Ancylostoma duodenale (common hookworm), Necator americanus (American hookworm) in single or mixed infections. Common adverse reactions include rash, abdominal pain, constipation, diarrhea and headache.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
The same dosage schedule applies to children and adults. The tablet may be chewed, swallowed, or crushed and mixed with food.
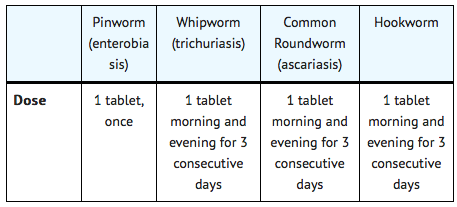
If the patient is not cured three weeks after treatment, a second course of treatment is advised. No special procedures, such as fasting or purging, are required.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Mebendazole in adult patients.
Non–Guideline-Supported Use
Capilaria Infection
Cestodes Infections
- Dosage
Filariasis
- Dosage: 300 mg/day PO for 28-45 days[4]
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)

Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Mebendazole in pediatric patients.
Non–Guideline-Supported Use
Capilaria Infection
Contraindications
Mebendazole is contraindicated in persons who have shown hypersensitivity to the drug.
Warnings
There is no evidence that mebendazole, even at high doses, is effective for hydatid disease. There have been rare reports of neutropenia and agranulocytosis when mebendazole was taken for prolonged periods and at dosages substantially above those recommended.
Adverse Reactions
Clinical Trials Experience
Gastrointestinal
- Abdominal pain
- Diarrhea in cases of massive infection and expulsion of worms.
Hypersensitivity
Central Nervous System
Liver
- Liver function test elevations AST (SGOT), ALT (SGPT), and GGT
- Hepatitis when mebendazole was taken for prolonged periods and at dosages substantially above those recommended.
Hematologic
Postmarketing Experience
There is limited information regarding Mebendazole Postmarketing Experience in the drug label.
Drug Interactions
- Preliminary evidence suggests that cimetidine inhibits mebendazole metabolism and may result in an increase in plasma concentrations of mebendazole.
Use in Specific Populations
Pregnancy
- Mebendazole has shown embryotoxic and teratogenic activity in pregnant rats at single oral doses as low as 10 mg/kg (approximately equal to the human dose, based on mg/m2). In view of these findings the use of mebendazole is not recommended in pregnant women. Although there are no adequate and well-controlled studies in pregnant women, a postmarketing survey has been done of a limited number of women who inadvertently had consumed mebendazole during the first trimester of pregnancy. The incidence of spontaneous abortion and malformation did not exceed that in the general population. In 170 deliveries on term, no teratogenic risk of mebendazole was identified.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Mebendazole in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Mebendazole during labor and delivery.
Nursing Mothers
- It is not known whether mebendazole is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when mebendazole is administered to a nursing woman.
Pediatric Use
- The drug has not been extensively studied in children under two years; therefore, in the treatment of children under two years the relative benefit/risk should be considered.
Geriatic Use
There is no FDA guidance on the use of Mebendazole in geriatric settings.
Gender
There is no FDA guidance on the use of Mebendazole with respect to specific gender populations.
Race
There is no FDA guidance on the use of Mebendazole with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Mebendazole in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Mebendazole in patients with hepatic impairment.
Females of Reproductive Potential and Males
- In carcinogenicity tests of mebendazole in mice and rats, no carcinogenic effects were seen at doses as high as 40 mg/kg (one to two times the human dose, based on mg/m2) given daily over two years. Dominant lethal mutation tests in mice showed no mutagenicity at single doses as high as 640 mg/kg (18 times the human dose, based on mg/m2). Neither the spermatocyte test, the F1 translocation test, nor the Ames test indicated mutagenic properties. Doses up to 40 mg/kg in mice (equal to the human dose, based on mg/m2), given to males for 60 days and to females for 14 days prior to gestation, had no effect upon fetuses and offspring, though there was slight maternal toxicity.
Immunocompromised Patients
There is no FDA guidance one the use of Mebendazole in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Mebendazole Administration in the drug label.
Monitoring
- Periodic assessment of organ system functions, including hematopoietic and hepatic, is advisable during prolonged therapy.
IV Compatibility
There is limited information regarding the compatibility of Mebendazole and IV administrations.
Overdosage
- In the event of accidental overdosage, gastrointestinal complaints lasting up to a few hours may occur. Vomiting and purging should be induced
Pharmacology
| |
Mebendazole
| |
| Systematic (IUPAC) name | |
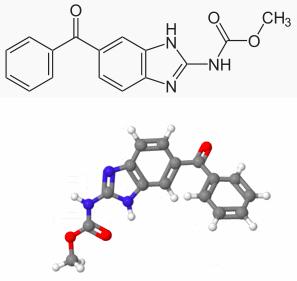
| methyl (5-benzoyl-1H-benzimidazol-2-yl)carbamate | |
| Identifiers | |
| CAS number | |
| ATC code | P02 Template:ATCvet |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 295.293 g/mol |
| SMILES | & |
| Physical data | |
| Melt. point | 288.5 °C (551 °F) |
| Pharmacokinetic data | |
| Bioavailability | 2-10% |
| Protein binding | 95% |
| Metabolism | Hepatic (extensive) |
| Half life | 3-6 hours |
| Excretion | Faeces, urine (5-10%) |
| Therapeutic considerations | |
| Pregnancy cat. | |
| Legal status |
Pharmacy Only (S2)(AU) ?(CA) GSL(UK) ?(US) |
| Routes | Oral |
Mechanism of Action
- Mebendazole inhibits the formation of the worms’ microtubules and causes the worms’ glucose depletion.
Structure
- Mebendazole is methyl 5-benzoylbenzimidazole-2-carbamate and has the following structural formula:

Pharmacodynamics
There is limited information regarding Mebendazole Pharmacodynamics in the drug label.
Pharmacokinetics
- Following administration of 100 mg twice daily for three consecutive days, plasma levels of mebendazole and its primary metabolite, the 2-amine, do not exceed 0.03 mcg/mL and 0.09 mcg/mL, respectively. All metabolites are devoid of anthelmintic activity. In man, approximately 2% of administered mebendazole is excreted in urine and the remainder in the feces as unchanged drug or a primary metabolite.
Nonclinical Toxicology
There is limited information regarding Mebendazole Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Mebendazole Clinical Studies in the drug label.
How Supplied
- Mebendazole 100 mg, chewable, round, light peach-colored, unscored tablets, debossed “93” and “107” on one side and plain on the other side, supplied in

Storage
Store at 20° to 25°C (68° to 77°F)
Images
Drug Images
{{#ask: Page Name::Mebendazole |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
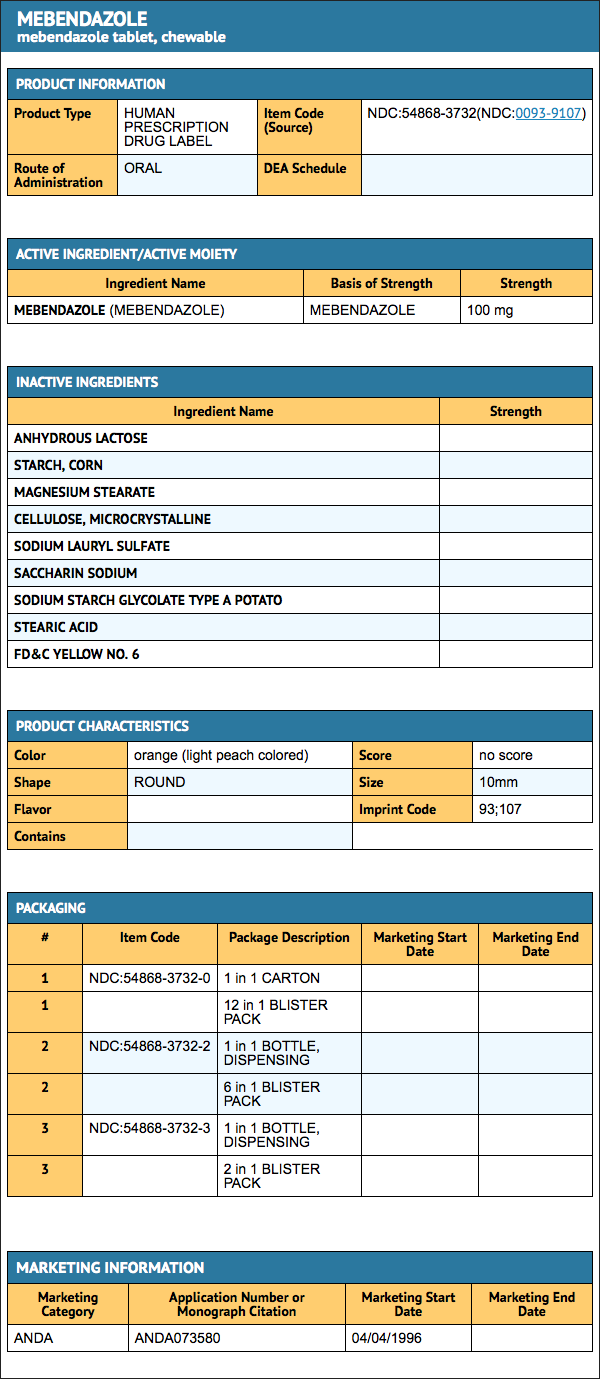
{{#ask: Label Page::Mebendazole |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Mebendazole Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Mebendazole interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Look-Alike Drug Names
There is limited information regarding Mebendazole Look-Alike Drug Names in the drug label.
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ 1.0 1.1 1.2 Keystone JS, Murdoch JK (1979). "Mebendazole". Ann Intern Med. 91 (4): 582–6. PMID 484964.
- ↑ 2.0 2.1 "Drugs for parasitic infections". Med Lett Drugs Ther. 34 (865): 17–26. 1992. PMID 1567506.
- ↑ Chavarria AP, Villarejos VM, Zeledón R (1977). "Mebendazole in the treatment of taeniasis solium and taeniasis saginata". Am J Trop Med Hyg. 26 (1): 118–20. PMID 842772.
- ↑ Van Hoegaerden M, Ivanoff B, Flocard F, Salle A, Chabaud B (1987). "The use of mebendazole in the treatment of filariases due to Loa loa and Mansonella perstans". Ann Trop Med Parasitol. 81 (3): 275–82. PMID 3478004.
{{#subobject:
|Page Name=Mebendazole
|Pill Name=Insert.png
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}