Varicella Zoster Immune Globulin
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sree Teja Yelamanchili, MBBS [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Varicella Zoster Immune Globulin is an immune serum that is FDA approved for the prophylaxis of post exposure varicella.. Common adverse reactions include injection site pain (overall, 2%; pregnant women, 9% ) and headache.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
Varicella Zoster Immune Globulin is indicated for post-exposure prophylaxis of varicella in high risk individuals. High risk groups include:
- immunocompromised children and adults
- newborns of mothers with varicella shortly before or after delivery
- premature infants
- neonates and infants less than one year of age
- adults without evidence of immunity
- pregnant women
Varicella Zoster Immune Globulin administration is intended to reduce the severity of varicella.
Administer Varicella Zoster Immune Globulin as soon as possible following varicella zoster virus (VZV) exposure, ideally within 96 hours for greatest effectiveness.
- There is no convincing evidence that Varicella Zoster Immune Globulin reduces the incidence of chickenpox infection after exposure to VZV.
- There is no convincing evidence that established infections with VZV can be modified by Varicella Zoster Immune Globulin administration.
- There is no indication for the prophylactic use of Varicella Zoster Immune Globulin in immunodeficient children or adults when there is a past history of varicella, unless the patient is undergoing bone marrow transplantation.
Dosage
- Dosing of Varicella Zoster Immune Globulin is based on body weight. Administer a single dose of Varicella Zoster Immune Globulin intramuscularly as recommended in TABLE 1.
- The minimum dose is 62.5 International Units (IU) for small infants under two kilograms body weight; the maximum dose of 625 IU should be administered for all patients greater than 40 kilograms in weight.
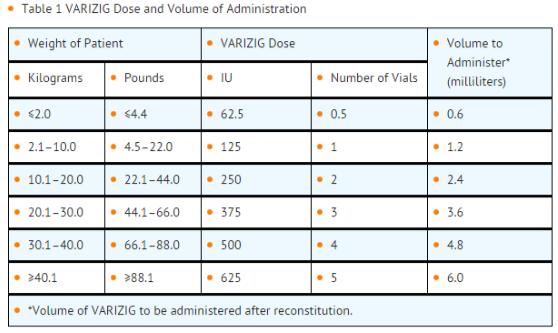
Consider a second full dose of Varicella Zoster Immune Globulin for high risk patients who have additional exposures to varicella greater than three weeks after initial Varicella Zoster Immune Globulin administration.
Reconstitution
Reconstitute Varicella Zoster Immune Globulin according to TABLE 2. Only use the accompanying Sterile Diluent with aseptic technique throughout. Reconstitute shortly before use.
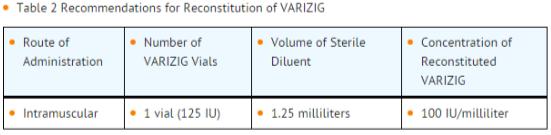
To reconstitute: Remove caps from the Sterile Diluent and Varicella Zoster Immune Globulin vials. Wipe exposed central portion of each rubber stopper with suitable disinfectant. Withdraw 1.25 milliliter of the Sterile Diluent using a suitable syringe and needle. Inject diluents slowly into the Varicella Zoster Immune Globulin vial at an angle so that the liquid is directed onto the inside glass wall of the vial containing the freeze-dried pellet. Wet pellet by gently tilting and inverting the vial. Avoid frothing. Gently swirl upright vial until dissolved (less than ten minutes). Do not shake. Inspect Varicella Zoster Immune Globulin visually for particulate matter and discoloration prior to administration. Do not use if turbid and/or discoloration is observed. Reconstituted product can be stored for up to 12 hours at 2 to 8°C (36 to 46ºF) prior to use. Do not freeze. Solutions that have been frozen should not be used. Varicella Zoster Immune Globulin is for single use only. Partially used vials, including the remaining Sterile Diluent, should be discarded.
Administration
- For intramuscular use only.
- Divide the intramuscular dose and administer in two or more injection sites, depending on patient size. Do not exceed 3 milliliters per injection site.
- Inject into the deltoid muscle or the anterolateral aspects of the upper thigh.
- Due to the risk of sciatic nerve injury, do not use the gluteal region as a routine injection site.
- If the gluteal region is used, only use the upper, outer quadrant.
- To prevent the transmission of infectious agents from one person to another, use a new disposable sterile syringe and needle for each individual patient.
Dosage forms and Strenghts
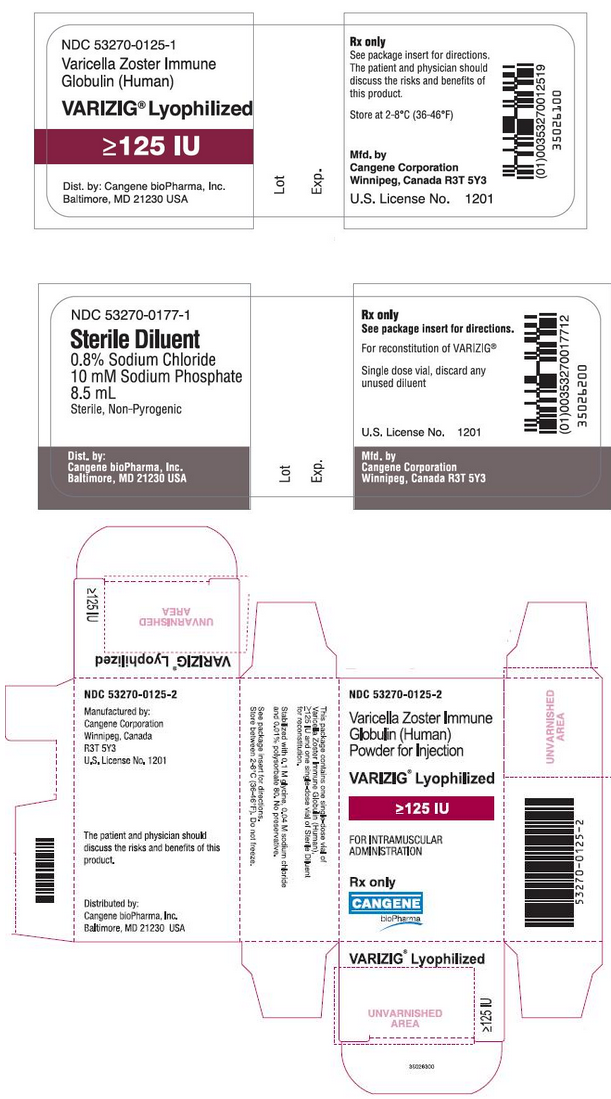
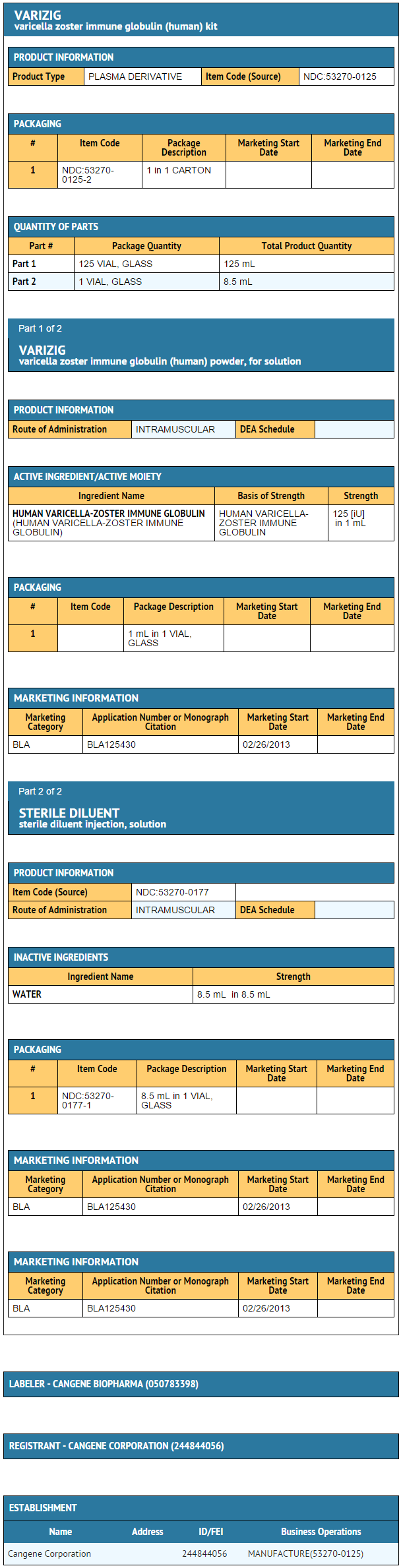
Varicella Zoster Immune Globulin is supplied as a sterile lyophilized powder for solution for intramuscular injection and is available in a single-use vial of 125 IU. Varicella Zoster Immune Globulin is accompanied by a vial containing 8.5 milliliters of Sterile Diluent for reconstitution. Each 125 IU vial of Varicella Zoster Immune Globulin contains less than 250 milligrams of total protein, mostly human immune globulin G (IgG). Varicella Zoster Immune Globulin contains no preservative and is intended for single use only. Varicella Zoster Immune Globulin does not contain mercury.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Varicella Zoster Immune Globulin in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Varicella Zoster Immune Globulin in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
The dosing recommendations in the treatment of pediatric patients are by body weight.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Varicella Zoster Immune Globulin in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Varicella Zoster Immune Globulin in pediatric patients.
Contraindications
- Individuals known to have anaphylactic or severe systemic (hypersensitivity) reactions to human immune globulin preparations should not receive Varicella Zoster Immune Globulin.
- IgA-deficient patients with antibodies against IgA and a history of hypersensitivity may have an anaphylactoid reaction.
- Varicella Zoster Immune Globulin contains less than 40 micrograms per milliliter of IgA.
Warnings
Thrombotic Events
Thrombotic events may occur during or following treatment with immune globulin products (1,2,3). Patients at risk include those with a history of atherosclerosis, multiple cardiovascular risk factors, advanced age, impaired cardiac output, coagulation disorders, prolonged periods of immobilization, and/or known/suspected hyperviscosity. Consider baseline assessment of blood viscosity in patients at risk for hyperviscosity including those with cryoglobulins, fasting chylomicronemia/markedly high triacylglycerols (triglycerides), or monoclonal gammopathies.
Coagulation Disorders
Administer Varicella Zoster Immune Globulin intramuscularly only. In patients who have severe thrombocytopenia or any coagulation disorder that would contraindicate intramuscular injections, only administer Varicella Zoster Immune Globulin if the expected benefits outweigh the potential risks.
Hypersensitivity
Severe hypersensitivity reactions may occur following Varicella Zoster Immune Globulin administration. Administer Varicella Zoster Immune Globulin in a setting with appropriate equipment, medication and personnel trained in the management of hypersensitivity, anaphylaxis and shock. In the case of hypersensitivity, discontinue administration of Varicella Zoster Immune Globulin immediately and provide appropriate treatment.
Varicella Zoster Immune Globulin contains trace amounts of IgA (less than 40 micrograms per milliliter). Patients with known antibodies to IgA have a greater risk of severe hypersensitivity and anaphylactic reactions. Varicella Zoster Immune Globulin is contraindicated in IgA deficient patients with antibodies against IgA and history of hypersensitivity reactions.
Transmissible Infectious Agents
Because Varicella Zoster Immune Globulin is made from human plasma, it may carry a risk of transmitting infectious agents, e.g., viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent. The plasma donors are screened for the presence of certain infectious agents and the manufacturing process for Varicella Zoster Immune Globulin includes measures to inactivate and remove certain viruses. Despite these measures, products derived from human plasma can still potentially transmit diseases. No cases of transmission of viral diseases, vCJD or CJD have been associated with the use of Varicella Zoster Immune Globulin.
Adverse Reactions
Clinical Trials Experience
The most common adverse drug reactions observed in clinical trials for all subjects and patients are the following:
- injection site pain (2%)
- headache (2%).
Less common adverse drug reactions reported include the following:
Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Three hundred and seventy seven high risk individuals received Varicella Zoster Immune Globulin intramuscularly in two clinical trials which included pregnant women, infants and immunocompromised pediatric and adult patients. The highest incidence of adverse reactions occurred in pregnant women (n=90), including injection site pain (9%), headache (4%), chills (2%) and fatigue (2%). All other adverse reactions occurred in 1% or less of clinical trial subjects within each high risk group. A single incidence of serum sickness (approximately one in 400 patients treated with Varicella Zoster Immune Globulin) was observed in an immunocompromised adolescent patient.
There were six reported adverse events related to the coagulation system (one deep vein thrombosis) in 372 subjects in the open-label, Expanded Access Protocol (EAP); the study was not designed to differentiate between adverse events attributed to the underlying medical condition and adverse reactions to Varicella Zoster Immune Globulin.
Postmarketing Experience
There is limited information regarding Varicella Zoster Immune Globulin Postmarketing Experience in the drug label.
Drug Interactions
The passive transfer of antibodies with immune globulin administration may impair the efficacy of live attenuated virus vaccines such as measles, rubella, mumps and varicella. Defer vaccination with live virus vaccines until approximately three months after Varicella Zoster Immune Globulin administration. Inform the immunizing physician of recent therapy with Varicella Zoster Immune Globulin so that appropriate measures can be taken.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
Pregnancy category C. Animal reproduction studies have not been conducted with Varicella Zoster Immune Globulin. It also is not known whether Varicella Zoster Immune Globulin can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Varicella Zoster Immune Globulin should be given to a pregnant woman only if clearly needed.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Varicella Zoster Immune Globulin in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Varicella Zoster Immune Globulin during labor and delivery.
Nursing Mothers
It is not known whether Varicella Zoster Immune Globulin is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Varicella Zoster Immune Globulin is administered to a nursing mother.
Pediatric Use
The safety and effectiveness of Varicella Zoster Immune Globulin have been evaluated for post-exposure prophylaxis in the Varicella Zoster Immune Globulin expanded access clinical trial in 265 pediatric patients, including immunocompromised pediatric patients: 70 preterm newborns and infants 38 term newborns 28 infants and toddlers 99 children and 30 adolescents. In the EAP, follow up data were available for 81 Varicella Zoster Immune Globulin treatments in 78 infants (including newborns, pre-term infants, and infants <1 year old). Two severe infections were reported, with pox count >100. One of these patients also developed probable varicella encephalitis.
Geriatic Use
- Clinical studies of Varicella Zoster Immune Globulin administered intramuscularly for post-exposure prophylaxis did not include sufficient numbers of geriatric subjects (aged 65 and over) to determine whether they respond differently from younger subjects.
- Use caution when administering Varicella Zoster Immune Globulin to patients age 65 and over who are judged to be at increased risk of thrombotic events. Do not exceed recommended doses and administer Varicella Zoster Immune Globulin intramuscularly only.
Gender
There is no FDA guidance on the use of Varicella Zoster Immune Globulin with respect to specific gender populations.
Race
There is no FDA guidance on the use of Varicella Zoster Immune Globulin with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Varicella Zoster Immune Globulin in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Varicella Zoster Immune Globulin in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Varicella Zoster Immune Globulin in women of reproductive potentials and males.
Immunocompromised Patients
In the EAP, eight immunocompromised subjects developed clinical varicella, and none developed varicella pneumonitis; however five are reported to have received concomitant cyclovir.
Administration and Monitoring
Administration
- Intramuscular use only
Monitoring
- Reduction in severity of clinical signs and symptoms of varicella zoster infection may indicate efficacy.
- Blood viscosity assessment; consider at baseline in patients at risk for hyperviscosity (eg, patients with cryoglobulins, fasting chylomicronemia, severe hypertriglyceridemia, or monoclonal gammopathies)
IV Compatibility
There is limited information regarding the compatibility of Varicella Zoster Immune Globulin and IV administrations.
Overdosage
Manifestations of an overdose of Varicella Zoster Immune Globulin administered intramuscularly are expected to be pain and tenderness at the injection site.
Pharmacology
There is limited information regarding Varicella Zoster Immune Globulin Pharmacology in the drug label.
Mechanism of Action
Varicella Zoster Immune Globulin provides passive immunization for non-immune individuals exposed to VZV, reducing the severity of varicella infections.
Structure
Varicella Zoster Immune Globulin is a solvent/detergent-treated sterile lyophilized preparation of purified human immune globulin G (IgG) containing antibodies to varicella zoster virus (anti-VZV). VZV is the causative agent of chickenpox. Varicella Zoster Immune Globulin is prepared from plasma donated by healthy, screened donors with high titers of antibodies to VZV, which is purified by an anion-exchange column chromatography manufacturing method. This donor selection process includes donors with high anti-VZV titers due to recent natural infection by VZV, or due to recurrent zoster infection (shingles).
Varicella Zoster Immune Globulin is supplied as a kit containing a single-use vial of Varicella Zoster Immune Globulin (lyophilized powder for solution for intramuscular injection with a potency of 125 IU) and a vial of 8.5 milliliters Sterile Diluent, which is used for reconstitution of the product prior to administration. Varicella Zoster Immune Globulin is intended for single use and should be administered intramuscularly.
The product potency is expressed in IU by comparison to the World Health Organization (WHO) international reference preparation for anti-VZV immune globulin. Each vial contains 125 IU of anti-VZV. The lyophilized Varicella Zoster Immune Globulin is formulated as 0.04 M sodium chloride, 0.1 M glycine and 0.01% polysorbate 80. The accompanying Sterile Diluent contains 0.8% sodium chloride and 10 mM sodium phosphate. The reconstituted Varicella Zoster Immune Globulin has a pH of 7 and contains no preservative.
Varicella Zoster Immune Globulin has not been tested for the presence of anti-Protein S antibodies that have been reported to arise transiently after VZV infection (4); however, it is assumed that the requirement that donors be healthy will alleviate this concern.
The source plasma used in the manufacture of this product was tested by FDA licensed nucleic acid testing (NAT) for human immunodeficiency virus-1 (HIV-1), hepatitis B virus (HBV) and hepatitis C virus (HCV) and found to be negative. Plasma also was tested by in-process NAT for hepatitis A virus (HAV) and parvovirus B19 (B19) via minipool testing; the limit for B19 in the manufacturing pool is set not to exceed 104 IU of B19 DNA per milliliter.
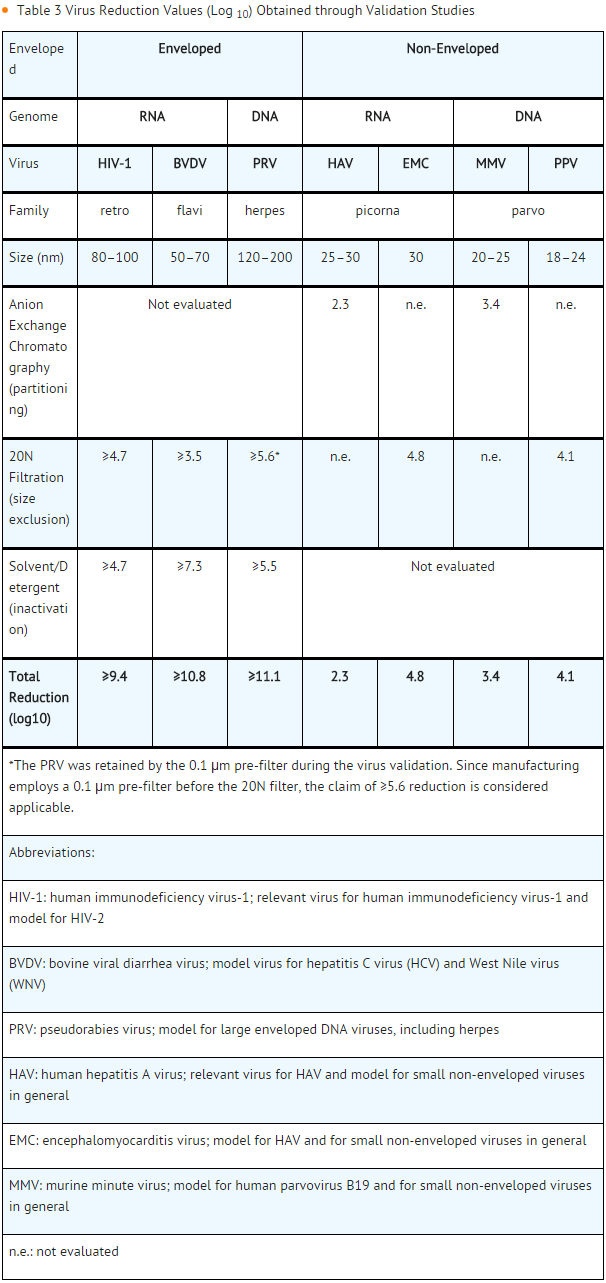
The manufacturing process contains two steps implemented specifically for virus clearance. The solvent/detergent step (using tri-n-butyl phosphate and Triton X-100) is effective in the inactivation of enveloped viruses, such as HBV, HCV and HIV-1. Virus filtration, using a Planova 20N virus filter, is effective for the removal of viruses based on their size, including some non-enveloped viruses. These two viral clearance steps are designed to increase product safety by reducing the risk of transmission of enveloped and non-enveloped viruses. In addition to these two specific steps, the process step of anion-exchange chromatography was identified as contributing to the overall viral clearance capacity for small non-enveloped viruses.
The inactivation and reduction of known enveloped and non-enveloped model viruses were validated in laboratory studies as summarized in TABLE 3. The viruses employed for spiking studies were selected to represent those viruses that are potential contaminants in the product, and to represent a wide range of physiochemical properties in order to challenge the manufacturing process’s ability for viral clearance in general.
Pharmacodynamics
There is limited information regarding Varicella Zoster Immune Globulin Pharmacodynamics in the drug label.
Pharmacokinetics
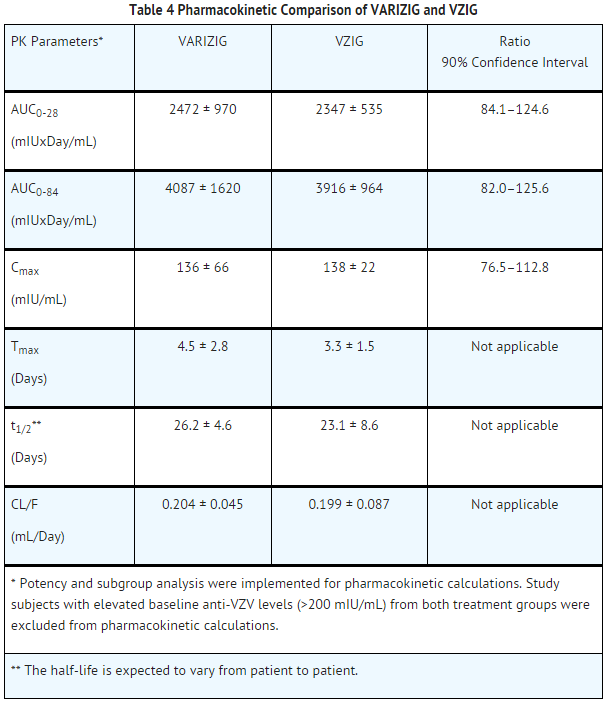
In a comparative pharmacokinetic clinical trial, 35 volunteers were administered an intramuscular dose of 12.5 IU/kg of Varicella Zoster Immune Globulin (n=18) or the comparator product VZIG™ (n=17). The dose of 12.5 IU/kg of VZIG or Varicella Zoster Immune Globulin given to the subjects was based on the assumption that the potency was similar for both products. For the bioequivalence analysis, a potency correction factor was applied (concentrations of Varicella Zoster Immune Globulin were multiplied by 2.3) to account for higher measured potency of the comparator product. The mean peak concentration (Cmax) of varicella antibodies occurred within five days of administration for both products. In the trial, baseline levels of anti-VZV antibodies ranged from 0 to 720 mIU/mL, therefore baseline levels were taken into account for pharmacokinetic calculations, to better represent the indicated population. After potency correction, baseline correction, and exclusion of subjects with baseline values of anti-VZV antibody levels of >200 mIU/mL, the two products were pharmacokinetically comparable.
Nonclinical Toxicology
There is limited information regarding Varicella Zoster Immune Globulin Nonclinical Toxicology in the drug label.
Clinical Studies
Pregnant Women Exposed to Varicella Zoster Virus
A randomized, open-label, multicenter, active controlled clinical trial was conducted in 60 pregnant women without immunity to VZV as confirmed by a latex agglutination test. Patients were stratified on the basis of time from first exposure to varicella as follows:
- one to four days post-exposure and
- five to 14 days post-exposure.
The women were randomized into one of three study arms as follows:
a single intravenous dose of 125 IU/10 kg body weight to a maximum dose of 625 IU of Varicella Zoster Immune Globulin a single intramuscular dose of 125 IU/10 kg body weight to a maximum dose of 625 IU of Varicella Zoster Immune Globulin, or a single intramuscular dose of 125 IU/10 kg body weight to a maximum dose of 625 IU of VZIG (licensed comparator product). Patients were followed for 42 days.
Incidence of clinical varicella was similar across all treatment groups with an overall incidence of 33%; however, in the subset of 28 subjects with more than 24 hours exposure to varicella, the incidence of clinical varicella in the combined treatment groups was 64%.
Mean weighted constitutional illness scores (CIS) (6) were similar across all groups and none of the subjects had serious complications of varicella. The small number of subjects in each treatment stratum and the lack of agreed upon pre-specified hypothesis testing precluded formal statistical comparisons between groups.
How Supplied
Varicella Zoster Immune Globulin is supplied as a kit in a carton box containing approximately 125 IUof anti-VZV supplied freeze-dried in a 6 mL type 1 glass tubing vial fitted with a 20 mm rubber lyophilization stopper and a 20 mm flip-off seal, one single dose vial of Sterile Diluent, non-pyrogenic for reconstitution of Varicella Zoster Immune Globulin and a package insert.
Storage
Store Varicella Zoster Immune Globulin at 2 to 8°C (36 to 46°F). Do not freeze. Do not use after expiration date. Use the product within 12 hours of reconstitution if stored at 2 to 8°C.
Images
Drug Images
{{#ask: Page Name::Varicella Zoster Immune Globulin |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Varicella Zoster Immune Globulin |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Inform patients of the following:
- Varicella Zoster Immune Globulin is intended to reduce the severity of chickenpox infections. Please see your doctor if you develop the signs and symptoms of varicella.
- Varicella Zoster Immune Globulin is prepared from human plasma and therefore, may contain infectious agents such as viruses that can cause disease.
- The risk that products derived from human plasma will transmit an infectious agent has been reduced by screening plasma donors for prior exposure to certain viruses, by testing for the presence of certain current virus infections, and by inactivating and/or removing certain viruses during manufacturing.
- Despite these measures, products derived from human plasma can still potentially transmit disease.
- There is also the possibility that unknown infectious agents may be present in such products.
- Tell patients that persons known to have severe, potentially life-threatening reactions to human immune globulin products should not receive Varicella Zoster Immune Globulin or any other immune globulin products unless the risk has been justified.
- Tell patients that persons who are deficient in IgA may have the potential for developing anti-IgA antibodies and have severe potentially life threatening allergic reactions.
- In the case of allergic or anaphylactic reaction, administration should be stopped immediately.
In the case of shock, the current medical standards for treatment of shock should be administered. Inform patients that administration of immune globulin may interfere with the response to live virus vaccines (e.g. measles, mumps, rubella and varicella), and instruct them to notify their immunizing physician of recent therapy with Varicella Zoster Immune Globulin.
Precautions with Alcohol
Alcohol-Varicella Zoster Immune Globulin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Varicella Zoster Immune Globulin
- Varicella Zoster Immune Globulin
Look-Alike Drug Names
There is limited information regarding Varicella Zoster Immune Globulin Look-Alike Drug Names in the drug label.
Price
References
The contents of this FDA label are provided by the National Library of Medicine.