Vancomycin dosage and administration
| Vancomycin |
|---|
| VANCOMYCIN HYDROCHLORIDE® FDA Package Insert |
| Description |
| Clinical Pharmacology |
| Microbiology |
| Indications and Usage |
| Contraindications |
| Warnings |
| Precautions |
| Adverse Reactions |
| Overdosage |
| Dosage and Administration |
| Directions For Use |
| How Supplied |
| Labels and Packages |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1];Associate Editor(s)-in-Chief: Abdurahman Khalil, M.D. [2]
Vancomycin Injection, USP in the GALAXY plastic container (PL 2040) is intended for intravenous use only.
Vancomycin in the GALAXY Container (PL 2040 Plastic) is not to be administered orally. An infusion rate of 10 mg/min or less is associated with fewer infusion-related events (see ADVERSE REACTIONS). Infusion related events may occur, however, at any rate or concentration.
Patients With Normal Renal Function
Adults
The usual daily intravenous dose is 2 g divided either as 500 mg every 6 hours or 1 g every 12 hours. Each dose should be administered at no more than 10 mg/min or over a period of at least 60 minutes, whichever is longer. Other patient factors, such as age or obesity, may call for modification of the usual intravenous daily dose.
Pediatric patients
The usual intravenous dosage of vancomycin is 10 mg/kg per dose given every 6 hours. Each dose should be administered over a period of at least 60 minutes. Close monitoring of serum concentrations of vancomycin may be warranted in these patients.
Neonates
In pediatric patients up to the age of 1 month, the total daily intravenous dosage may be lower. In neonates, an initial dose of 15 mg/kg is suggested, followed by 10 mg/kg every 12 hours for neonates in the 1st week of life and every 8 hours thereafter up to the age of 1 month. Each dose should be administered over 60 minutes. In premature infants, vancomycin clearance decreases as postconceptional age decreases. Therefore, longer dosing intervals may be necessary in premature infants. Close monitoring of serum concentrations of vancomycin is recommended in these patients.
Patients With Impaired Renal Function and Elderly Patients
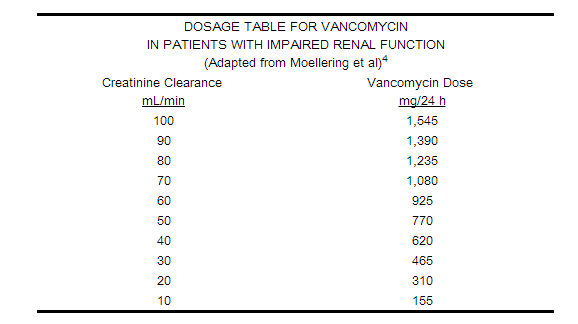
Dosage adjustment must be made in patients with impaired renal function. In the elderly, greater dosage reductions than expected may be necessary because of decreased renal function. Measurement of vancomycin serum concentrations can be helpful in optimizing therapy, especially in seriously ill patients with changing renal function. Vancomycin serum concentrations can be determined by use of microbiologic assay, radioimmunoassay, fluorescence polarization immunoassay, fluorescence immunoassay, or high-pressure liquid chromatography. If creatinine clearance can be measured or estimated accurately, the dosage for most patients with renal impairment can be calculated using the following table. The dosage of vancomycin per day in mg is about 15 times the glomerular filtration rate in mL/min:
The initial dose should be no less than 15 mg/kg, even in patients with mild to moderate renal insufficiency. The table is not valid for functionally anephric patients. For such patients, an initial dose of 15 mg/kg of body weight should be given to achieve prompt therapeutic serum concentrations. The dose required to maintain stable concentrations is 1.9 mg/kg/24 h. In patients with marked renal impairment, it may be more convenient to give maintenance doses of 250 to 1,000 mg once every several days rather than administering the drug on a daily basis. In anuria, a dose of 1,000 mg every 7 to 10 days has been recommended.
When only the serum creatinine concentration is known, the following formula (based on sex, weight, and age of the patient) may be used to calculate creatinine clearance. Calculated creatinine clearances (mL/min) are only estimates. The creatinine clearance should be measured promptly.
The serum creatinine must represent a steady state of renal function. Otherwise, the estimated value for creatinine clearance is not valid. Such a calculated clearance is an overestimate of actual clearance in patients with conditions: (1) characterized by decreasing renal function, such as shock, severe heart failure, or oliguria; (2) in which a normal relationship between muscle mass and total body weight is not present, such as obese patients or those with liver disease, edema, or ascites; and (3) accompanied by debilitation, malnutrition, or inactivity. The safety and efficacy of vancomycin administration by the intrathecal (intralumbar or intraventricular) routes have not been established.
Intermittent infusion is the recommended method of administration.
References
http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/050671s014lbl.pdf