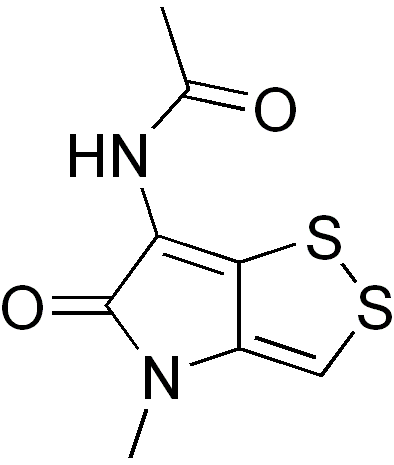
Thiolutin
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C8H8N2O2S2 |
| Molar mass | 228.293 g/mol |
|
WikiDoc Resources for Thiolutin |
|
Articles |
|---|
|
Most recent articles on Thiolutin |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Thiolutin at Clinical Trials.gov Clinical Trials on Thiolutin at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Thiolutin
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Thiolutin Discussion groups on Thiolutin Directions to Hospitals Treating Thiolutin Risk calculators and risk factors for Thiolutin
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Thiolutin |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Thiolutin is a sulfur-containing antibiotic, which is a potent inhibitor of bacterial and yeast RNA polymerases. It was found to inhibit in vitro RNA synthesis directed by all three yeast RNA polymerases (I, II, and III). Thiolutin is also an inhibitor of mannan and glucan formation in Saccharomyces cerevisiae and used for the analysis of mRNA stability. Studies have shown that thiolutin inhibits adhesion of human umbilical vein endothelial cells (HUVECs) to vitronectin and thus suppresses tumor cell-induced angiogenesis in vivo.
Thiolutin is formed in submerged fermentation by several strains of Streptomycetes (source: Fermentek product page)
Synonyms: farcinicin, propiopyvothine, acetopyrrothine
Some sources erroneously specify "aureothricin" as a synonym of thiolutin. Aureothricin is an antibiotic very similar to Thiolutin, and is created as a by-product during the Thiolutin fermentation.
References
- Pages with script errors
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs missing an ATC code
- Drugs with no legal status
- Articles containing unverified chemical infoboxes
- Antibiotics
- Organic disulfides