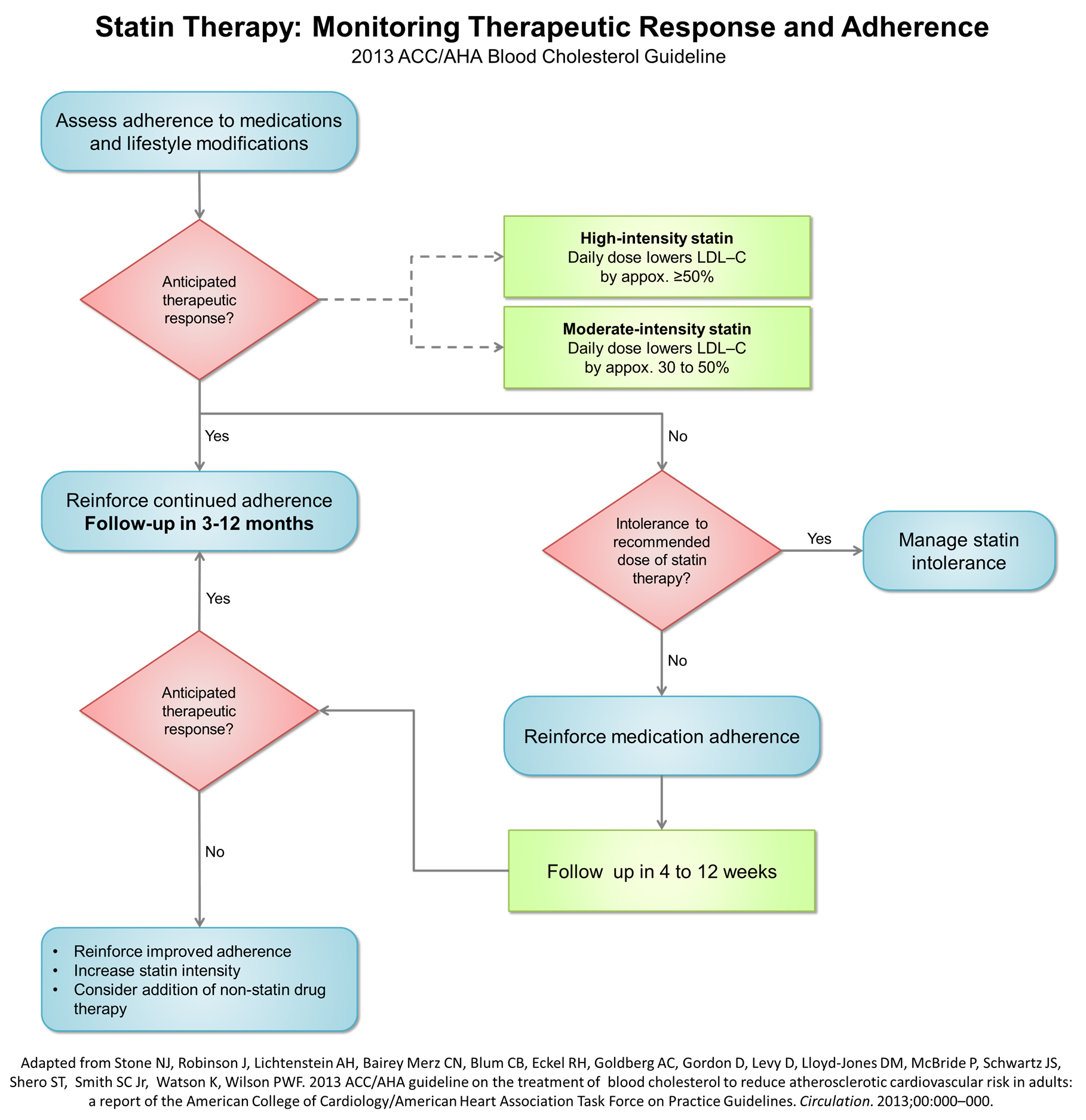
Therapeutic response to statin therapy
Template:Hypercholesterolemia Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
2013 ACC AHA guidelin Recommendations for Monitoring, Optimizing, and Addressing Insufficient Response to Statin Therapy
Monitoring Statin Therapy
| Class I |
| "1. Adherence to medication and lifestyle, therapeutic response to statin therapy, and safety should be regularly assessed. This should also include a fasting lipid panel performed within 4 to 12 weeks after initiation or dose adjustment, and every 3 to 12 months thereafter. Other safety measurements should be measured as clinically indicated.[1] |
Optimizing Statin Therapy
| Class I* |
| "1. The maximum tolerated intensity of statin should be used in individuals for whom a high- or moderate-intensity statin is recommended, but not tolerated.[2][3][4][1] (Level of Evidence: B)" |
"*Several RCTs found that low and low-moderate intensity statin therapy reduced ASCVD events. In addition, the CTT meta-analyses found each 39 mg/dL reduction in LDL–C reduces ASCVD risk by 22%. Therefore, the Panel considered that submaximal statin therapy should be used to reduce ASCVD risk in those unable to tolerate moderate-or high-intensity statin therapy."
Insufficient Response to Statin Therapy
| Class I |
"1. In individuals who have a less-than-anticipated therapeutic response or are intolerant of the recommended intensity of statin therapy, the following should be performed[1]:
|
| Class IIa |
"1. It is reasonable to use the following as indicators of anticipated therapeutic response to the recommended intensity of statin therapy. Focus is on the intensity of the statin therapy. As an aid to monitoring[5][6][7][8][9][10]:
|
| "2. In individuals who are candidates for statin treatment but are completely statin intolerant, it is reasonable to use nonstatin cholesterol-lowering drugs that have been shown to reduce ASCVD events in RCTs if the ASCVD risk-reduction benefits outweigh the potential for adverse effects.[11][12][13][14][15][16][17][18](Level of Evidence: B)" |
| Class IIb |
| "1. In individuals at higher ASCVD risk receiving the maximum tolerated intensity of statin therapy who continue to have a less-than-anticipated therapeutic response, addition of a non-statin cholesterol-lowering drug(s) may be considered if the ASCVD risk-reduction benefits outweigh the potential for adverse effects.
Higher-risk individuals include[19][20][21][22][23]:
Preference should be given to non-statin cholesterol-lowering drugs shown to reduce ASCVD events in RCTs.(Level of Evidence: C)" |
"†In those already on a statin, in whom baseline LDL–C is unknown, an LDL–C <100 mg/dL was observed in most individuals receiving high-intensity statin therapy.
‡Clinical ASCVD includes acute coronary syndromes, or a history of MI, stable or unstable angina, coronary or other arterial revascularization, stroke, TIA, or peripheral arterial disease presumed to be of atherosclerotic origin."
References
- ↑ 1.0 1.1 1.2 Miller M, Stone NJ, Ballantyne C, Bittner V, Criqui MH, Ginsberg HN; et al. (2011). "Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association". Circulation. 123 (20): 2292–333. doi:10.1161/CIR.0b013e3182160726. PMID 21502576.
- ↑ Fried TR, Tinetti ME, Towle V, O'Leary JR, Iannone L (2011). "Effects of benefits and harms on older persons' willingness to take medication for primary cardiovascular prevention". Arch Intern Med. 171 (10): 923–8. doi:10.1001/archinternmed.2011.32. PMC 3101287. PMID 21357797.
- ↑ Robinson JG, Bakris G, Torner J, Stone NJ, Wallace R (2007). "Is it time for a cardiovascular primary prevention trial in the elderly?". Stroke. 38 (2): 441–50. doi:10.1161/01.STR.0000254602.58896.d2. PMID 17194877.
- ↑ Porock D, Oliver DP, Zweig S, Rantz M, Mehr D, Madsen R; et al. (2005). "Predicting death in the nursing home: development and validation of the 6-month Minimum Data Set mortality risk index". J Gerontol A Biol Sci Med Sci. 60 (4): 491–8. PMID 15933390.
- ↑ LaRosa JC, Grundy SM, Waters DD, Shear C, Barter P, Fruchart JC; et al. (2005). "Intensive lipid lowering with atorvastatin in patients with stable coronary disease". N Engl J Med. 352 (14): 1425–35. doi:10.1056/NEJMoa050461. PMID 15755765.Review in: ACP J Club. 2005 Sep-Oct;143(2):38
- ↑ Pedersen TR, Faergeman O, Kastelein JJ, Olsson AG, Tikkanen MJ, Holme I; et al. (2005). "High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial". JAMA. 294 (19): 2437–45. doi:10.1001/jama.294.19.2437. PMID 16287954.
- ↑ Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R; et al. (2004). "Intensive versus moderate lipid lowering with statins after acute coronary syndromes". N Engl J Med. 350 (15): 1495–504. doi:10.1056/NEJMoa040583. PMID 15007110.Review in: ACP J Club. 2004 Sep-Oct;141(2):33
- ↑ Amarenco P, Bogousslavsky J, Callahan A, Goldstein LB, Hennerici M, Rudolph AE; et al. (2006). "High-dose atorvastatin after stroke or transient ischemic attack". N Engl J Med. 355 (6): 549–59. doi:10.1056/NEJMoa061894. PMID 16899775.Review in: ACP J Club. 2007 Jan-Feb;146(1):7
- ↑ Thompson GR, HEART-UK LDL Apheresis Working Group (2008). "Recommendations for the use of LDL apheresis". Atherosclerosis. 198 (2): 247–55. doi:10.1016/j.atherosclerosis.2008.02.009. PMID 18371971.
- ↑ Schwertz DW, Badellino KO (2008). "High-dose statin therapy for secondary prevention of stroke: stroke prevention by aggressive reduction in cholesterol levels study review". J Cardiovasc Nurs. 23 (1): 8–13. doi:10.1097/01.JCN.0000305061.88624.fc. PMID 18158500.
- ↑ Eckel RH (2010). "Approach to the patient who is intolerant of statin therapy". J Clin Endocrinol Metab. 95 (5): 2015–22. doi:10.1210/jc.2009-2689. PMID 20444930.
- ↑ "Clofibrate and niacin in coronary heart disease". JAMA. 231 (4): 360–81. 1975. PMID 1088963.
- ↑ Frick MH, Elo O, Haapa K, Heinonen OP, Heinsalmi P, Helo P; et al. (1987). "Helsinki Heart Study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease". N Engl J Med. 317 (20): 1237–45. doi:10.1056/NEJM198711123172001. PMID 3313041.
- ↑ "The Lipid Research Clinics Coronary Primary Prevention Trial results. II. The relationship of reduction in incidence of coronary heart disease to cholesterol lowering". JAMA. 251 (3): 365–74. 1984. PMID 6361300.
- ↑ "The Lipid Research Clinics Coronary Primary Prevention Trial results. I. Reduction in incidence of coronary heart disease". JAMA. 251 (3): 351–64. 1984. PMID 6361299.
- ↑ Rubins HB, Robins SJ, Collins D, Fye CL, Anderson JW, Elam MB; et al. (1999). "Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group". N Engl J Med. 341 (6): 410–8. doi:10.1056/NEJM199908053410604. PMID 10438259.
- ↑ Keech A, Simes RJ, Barter P, Best J, Scott R, Taskinen MR; et al. (2005). "Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial". Lancet. 366 (9500): 1849–61. doi:10.1016/S0140-6736(05)67667-2. PMID 16310551.Review in: ACP J Club. 2006 May-Jun;144(3):65 Review in: Evid Based Med. 2006 Jun;11(3):86
- ↑ HPS2-THRIVE Collaborative Group (2013). "HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment". Eur Heart J. 34 (17): 1279–91. doi:10.1093/eurheartj/eht055. PMC 3640201. PMID 23444397.
- ↑ AIM-HIGH Investigators. Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P; et al. (2011). "Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy". N Engl J Med. 365 (24): 2255–67. doi:10.1056/NEJMoa1107579. PMID 22085343.Review in: Ann Intern Med. 2012 Apr 17;156(8):JC4-08
- ↑ ACCORD Study Group. Ginsberg HN, Elam MB, Lovato LC, Crouse JR, Leiter LA; et al. (2010). "Effects of combination lipid therapy in type 2 diabetes mellitus". N Engl J Med. 362 (17): 1563–74. doi:10.1056/NEJMoa1001282. PMC 2879499. PMID 20228404.Review in: J Fam Pract. 2010 Oct;59(10):582-4 Review in: Ann Intern Med. 2010 Jul 20;153(2):JC1-5
- ↑ Rossebø AB, Pedersen TR, Allen C, Boman K, Chambers J, Egstrup K; et al. (2007). "Design and baseline characteristics of the simvastatin and ezetimibe in aortic stenosis (SEAS) study". Am J Cardiol. 99 (7): 970–3. doi:10.1016/j.amjcard.2006.10.064. PMID 17398194.
- ↑ "Study of Heart and Renal Protection (SHARP): randomized trial to assess the effects of lowering low-density lipoprotein cholesterol among 9,438 patients with chronic kidney disease". Am Heart J. 160 (5): 785–794.e10. 2010. doi:10.1016/j.ahj.2010.08.012. PMID 21095263.
- ↑ Yokoyama M, Origasa H, JELIS Investigators (2003). "Effects of eicosapentaenoic acid on cardiovascular events in Japanese patients with hypercholesterolemia: rationale, design, and baseline characteristics of the Japan EPA Lipid Intervention Study (JELIS)". Am Heart J. 146 (4): 613–20. doi:10.1016/S0002-8703(03)00367-3. PMID 14564313.