Rifampin microbiology
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Chetan Lokhande, M.B.B.S [2]
Microbiology
Mechanism of Action
Rifampin inhibits DNA-dependent RNA polymerase activity in susceptible Mycobacterium tuberculosis organisms. Specifically, it interacts with bacterial RNA polymerase but does not inhibit the mammalian enzyme.
Drug Resistance
Organisms resistant to rifampin are likely to be resistant to other rifamycins. In the treatment of both tuberculosis and the meningococcal carrier state (see Indications And Usage), the small number of resistant cells present within large populations of susceptible cells can rapidly become predominant. In addition, resistance to rifampin has been determined to occur as single-step mutations of the DNA-dependent RNA polymerase. Since resistance can emerge rapidly, appropriate susceptibility tests should be performed in the event of persistent positive cultures.
Activity in vitro and in vivo
Rifampin has bactericidal activity in vitro against slow and intermittently growing M tuberculosis organisms.
Rifampin has been shown to be active against most strains of the following microorganisms, both in vitro and in clinical infections as described in the Indications And Usage section.
Aerobic Gram-Negative Microorganisms
- Neisseria meningitidis
"Other" Microorganisms
- Mycobacterium tuberculosis
The following in vitro data are available, but their clinical significance is unknown.
Rifampin exhibits in vitro activity against most strains of the following microorganisms; however, the safety and effectiveness of rifampin in treating clinical infections due to these microorganisms have not been established in adequate and well-controlled trials.
Aerobic Gram-Positive Microorganisms
- Staphylococcus aureus (including Methicillin-Resistant S. aureus/MRSA)
- Staphylococcus epidermidis
Aerobic Gram-Negative Microorganisms
- Haemophilus influenzae
"Other" Microorganisms
- Mycobacterium leprae
β-lactamase production should have no effect on rifampin activity.
Susceptibility Testing
Prior to initiation of therapy, appropriate specimens should be collected for identification of the infecting organism and in vitro susceptibility tests.
In vitro testing for Mycobacterium tuberculosis isolates:
Two standardized in vitro susceptibility methods are available for testing rifampin against M tuberculosis organisms. The agar proportion method (CDC or CLSI(1) M24-A) utilizes Middlebrook 7H10 medium impregnated with rifampin at a final concentration of 1.0 mcg/mL to determine drug resistance. After three weeks of incubation MIC99 values are calculated by comparing the quantity of organisms growing in the medium containing drug to the control cultures. Mycobacterial growth in the presence of drug, of at least 1% of the growth in the control culture, indicates resistance.
The radiometric broth method employs the BACTEC 460 machine to compare the growth index from untreated control cultures to cultures grown in the presence of 2.0 mcg/mL of rifampin. Strict adherence to the manufacturer's instructions for sample processing and data interpretation is required for this assay.
Susceptibility test results obtained by the two different methods can only be compared if the appropriate rifampin concentration is used for each test method as indicated above. Both procedures require the use of M tuberculosis H37Rv ATCC 27294 as a control organism.
The clinical relevance of in vitro susceptibility test results for mycobacterial species other than M tuberculosis using either the radiometric or the proportion method has not been determined.
In vitro testing for Neisseria meningitidis isolates:
Dilution Techniques
Quantitative methods that are used to determine minimum inhibitory concentrations provide reproducible estimates of the susceptibility of bacteria to antimicrobial compounds. One such standardized procedure uses a standardized dilution method2,4 (broth, agar, or microdilution) or equivalent with rifampin powder. The MIC values obtained should be interpreted according to the following criteria for Neisseria meningitidis:
 |
A report of "susceptible" indicates that the pathogen is likely to be inhibited by usually achievable concentrations of the antimicrobial compound in the blood. A report of "intermediate" indicates that the result should be considered equivocal, and if the microorganism is not fully susceptible to alternative, clinically feasible drugs, the test should be repeated. This category implies possible clinical applicability in body sites where the drug is physiologically concentrated or in situations where the maximum acceptable dose of drug can be used. This category also provides a buffer zone that prevents small-uncontrolled technical factors from causing major discrepancies in interpretation. A report of "resistant" indicates that usually achievable concentrations of the antimicrobial compound in the blood are unlikely to be inhibitory and that other therapy should be selected.
Measurement of MIC or minimum bactericidal concentrations (MBC) and achieved antimicrobial compound concentrations may be appropriate to guide therapy in some infections. (See Clinical Pharmacology section for further information on drug concentrations achieved in infected body sites and other pharmacokinetic properties of this antimicrobial drug product.)
Standardized susceptibility test procedures require the use of laboratory control microorganisms. The use of these microorganisms does not imply clinical efficacy (see Indications And Usage); they are used to control the technical aspects of the laboratory procedures. Standard rifampin powder should give the following MIC values:
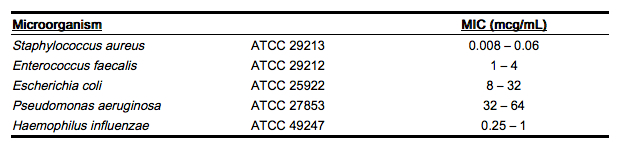 |
Diffusion Techniques
Quantitative methods that require measurement of zone diameters provide reproducible estimates of the susceptibility of bacteria to antimicrobial compounds. One such standardized procedure3,4 that has been recommended for use with disks to test the susceptibility of microorganisms to rifampin uses the 5 mcg rifampin disk. Interpretation involves correlation of the diameter obtained in the disk test with the MIC for rifampin.
Reports from the laboratory providing results of the standard single-disk susceptibility test with a 5 mcg rifampin disk should be interpreted according to the following criteria for Neisseria meningitidis:
 |
Interpretation should be as stated above for results using dilution techniques.
As with standard dilution techniques, diffusion methods require the use of laboratory control microorganisms. The use of these microorganisms does not imply clinical efficacy (see INdications And Usage); they are used to control the technical aspects of the laboratory procedures. The 5 mcg rifampin disk should provide the following zone diameters in these quality control strains:[1]
 |
References
Adapted from the FDA Package Insert.